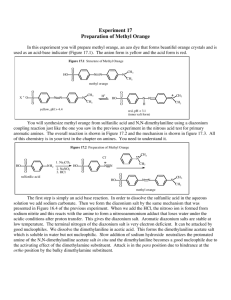
Synthesis of methyl orange in a micro reactor Hamburger, L.J. and
advertisement

Synthesis of methyl orange in a micro reactor Hamburger, L.J. and Koorn, I. Huygens College, Heerhugowaard, the Netherlands May 2012 Summary The production of methyl orange in a micro reactor. When will there be the highest flow rate of methyl orange? The variables that could be controlled were temperature and flow rate. The results for the highest methyl orange production: put the flow rate to a low speed and set the temperature at 80 degrees Celsius. Introduction Methyl orange is an azo-compound, what is used as a PH meter in titrations and too dye and print textile. Producing methyl orange on a big scale, the temperature needs to be kept low, because of risk of explosion. This test is carried out on a smaller scale so the production of methyl orange in a micro reactor makes it possible to let the temperature rise to a higher value than producing on big scale. this produces the methyl orange, see figure 3. Figure 3. (3) This raises the question: At which temperature and which flow rate will the the highest concentration of methyl orange be reached? Figure 1. (1) Methyl orange is produced by the reaction of sulfanilic acid, sodium nitrite, and dimethylaniline. First, sulfanilic acid is dissolved in dilute aqueous acid. Sodium nitrite is added to produce a diazonium salt see figure 2. Figure 2. (2) After the diazonium salt is produced, the salt is coupled with N,N-dimethylaniline, Our hypothesis is: a high temperature and a low flow rate will give us a maximum concentration of methyl orange. Expecting that a high temperature will be better than a low temperature, because particles collide harder in each other at higher temperature than at low temperatures. The higher temperature makes particles move faster so there are more collisions and they collide harder.(4) The use of a low flow rate is better because there will be more time for the reaction to take place. Experimental procedure and approach The experiment was done in a micro reactor which has 3 inlets. Two inlets are used to inject the solution to make methyl orange. The third inlet will stop the reaction of methyl orange, after a spectrometer and a photoelectric meter will measure the concentration of methyl orange. Inlet 1 contains a solution of sodium carbonate, sulfanic acid and sodium nitrate. Inlet 2 contains a solution of N,N-dimethylaniline and hydrochloric acid. Inlet 3 contains a solution of sodium hydroxide. Those concentrations were not changeable. The changeable variables were the flow rate and the temperature. The flow rate could be varied between 1 µl/min and 1500 µl/min. The temperature in the reactor was changeable between 8 degrees Celsius and 80 degrees Celsius. Each experiment was done twice and if necessary the measurements were done three times to make sure the measurements were reliable. Measuring the best temperature was done at a flow rate of 100 µl/min. The temperature was varied with steps of 20 degrees Celsius. The results lead to a best temperature of 80 degrees Celsius. The optimum flow rate had to be found to get the highest production of methyl orange. The flow rate was varied from 50 µl/min to 800 µl/min. Results Temperature in Celsius Concentration of methyl orange in M 20 0,000073 40 0,000333 60 0,000555 80 0,000611 Flow rate in µl/min Concentration of methyl orange in M 50 0,000705 100 0,000612 200 0,000495 400 0,00039 800 0,0003 ± 0,00002 Looking at the results, the temperature must be set at 80 degrees Celsius. The flow rate looks like to be a nearly straight line, when you keep on doubling the flow rate. It cannot be concluded that it will continue like a straight line. In this graphic the straight line indicates the highest concentration of methyl orange will be at an optimum at to lowest flow rate that is possible. The highest production of methyl orange would be at a flow rate around 1600 µl/min. Conclusion and discussion Concluded is that a low speed will give more methyl orange. The temperature must be set at 80 degrees to get the highest concentration of methyl orange. In combination with the lowest possible flow rate. So recommend is to do the experiment at the temperature close to 80 degrees Celsius. The measurement of the concentration of methyl orange at a low flow rate doesn't work as it should work it the graph starts to jump from 0,0007 M to 0,002 M resulting in unreliable measurements. The high speed measurements are less reliable, because there was no possibility to fill the inlets with more than 1 ml. So it was needed to stop quiet fast with the measurement before it would shoot of the scale. Bibliography 1. Methyl orange, http://en.wikipedia.org/wiki/File:Methylorange-sample.jp 2. Synthesis of diazonium salt http://antoine.frostburg.edu/chem/senese /101/index.shtml 3. Synthesis of methyl orange, http://antoine.frostburg.edu/chem/ senese/101/index.shtml 4. Fourth class chemistry book