Tijl and Huck
advertisement
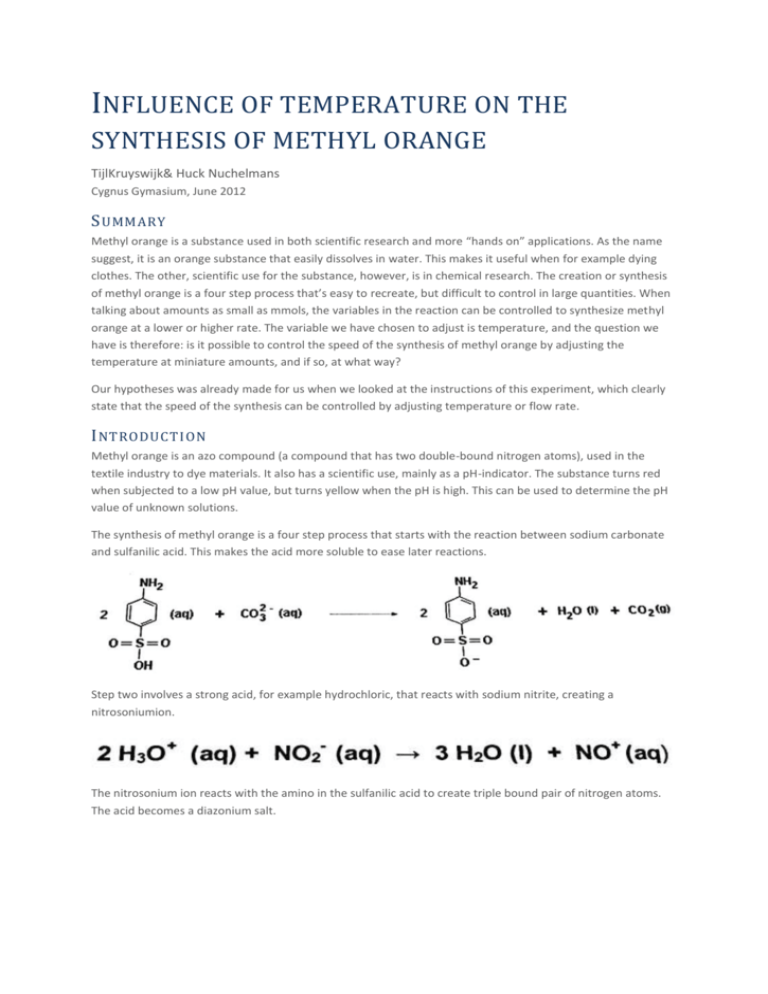
INFLUENCE OF TEMPERATURE ON THE SYNTHESIS OF METHYL ORANGE TijlKruyswijk& Huck Nuchelmans Cygnus Gymasium, June 2012 S UMMARY Methyl orange is a substance used in both scientific research and more “hands on” applications. As the name suggest, it is an orange substance that easily dissolves in water. This makes it useful when for example dying clothes. The other, scientific use for the substance, however, is in chemical research. The creation or synthesis of methyl orange is a four step process that’s easy to recreate, but difficult to control in large quantities. When talking about amounts as small as mmols, the variables in the reaction can be controlled to synthesize methyl orange at a lower or higher rate. The variable we have chosen to adjust is temperature, and the question we have is therefore: is it possible to control the speed of the synthesis of methyl orange by adjusting the temperature at miniature amounts, and if so, at what way? Our hypotheses was already made for us when we looked at the instructions of this experiment, which clearly state that the speed of the synthesis can be controlled by adjusting temperature or flow rate. I NTRODUCTION Methyl orange is an azo compound (a compound that has two double-bound nitrogen atoms), used in the textile industry to dye materials. It also has a scientific use, mainly as a pH-indicator. The substance turns red when subjected to a low pH value, but turns yellow when the pH is high. This can be used to determine the pH value of unknown solutions. The synthesis of methyl orange is a four step process that starts with the reaction between sodium carbonate and sulfanilic acid. This makes the acid more soluble to ease later reactions. Step two involves a strong acid, for example hydrochloric, that reacts with sodium nitrite, creating a nitrosoniumion. The nitrosonium ion reacts with the amino in the sulfanilic acid to create triple bound pair of nitrogen atoms. The acid becomes a diazonium salt. In the final part of the synthesis the salt reacts with an addes N,N-dimethylanaline and a hydroxide ion to create methyl orange. Note that each of these reactions also have water as a by-product. Since the synthesis of methyl orange is an exothermic reaction, it requires large amounts of cooling on a large scale to prevent overheating the compounds. This makes adjusting temperature difficult, but at tiny amounts of compounds, the energy released from the reaction is so small that temperature can easily be regulated to get different outcomes. E XPERIMENT The experiment was set up as such: a microreactor had three inlets, one outlet and a long channel to allow for the reaction to take place. Each of the inlets had a reagent for the synthesis (sulfanilic acid + sodium nitrite, N,N-dimethylanaline+ hydrochloric acid and lye). Inlet 1 and 2 could also be filled with with alcohol to clean the reactor after a measurement cycle. Each of the inlets had an adjustable flow rate (ranging from 1 µmol/min to 1500µmol/min). The entire reactor could either be heated up or cooled down using an adjustable temperature (ranging from 8°C to 80°C). When each of the reagents was released at a predetermined flow rate (we chose 300µmol/min), a spectrometer in an Erlenmeyer measured the amount of methyl orange that was being produced. This data was made into a graph, which clearly showed the concentration of methyl orange per time unit. Eventually, the value would become a constant as no more methyl orange was being made, and the measurement could be stopped. The graph data gets saved and the reactor is again ready to be cleaned with alcohol. R ESULTS The results of our experiment, where we chose to measure the speed at which methyl orange was formed under different temperatures, we got the graph shown below. All flow rates were the same, so no significant changes in colour was observed during the reaction (which means, the reactor got the standard brown-yellow colour). During the cleaning, however, the colour very briefly shifted to yellow since alcohol has base-like components. Note that when saving the data, something went wrong, and now our measurements for 60°C and 40°C are exactly the same. Concentration of methyl orange in mmol/L Time in seconds We added a standard logarithmic graph next to our measurements to show that the correlation between time and concentration is in fact logarithmic. This graph shows that the temperature had a significant impact on the speed at which methyl orange was created. The amount created at 80°C is nearly 20 times higher than that of 20°C. The stable point in the graph was also much less stable at higher temperatures, however, indicating that the reaction was less consistent at the higher temperatures. C ONCLUSION AND DISCUS SION Our results show that a microreaction is indeed possible, and that the speed of said reaction can be altered by adjusting the temperature of the reactor. However, a higher temperature also appears to mean less stable, so a safe zone in temperature must still be picked. We have no idea how much methyl orange we ended up making, though.