Water

SBI 3UC Topic 3: The Chemistry of Life
The Nature of Molecules
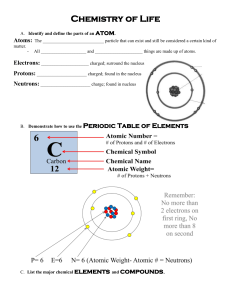
ATOMS: basic unit of all matter - neutral charge results when the proton # = electron #.
Protons (+) and neutrons (0) in nucleus
Electrons (-) in orbits around nucleus
Atomic number = number of protons
Atomic mass = mass of protons + neutrons
Subatomic particles seen indirectly via collisions
Charge Neutral atoms: electron # = proton #
4 types of atoms make up 97% of all living systems;
Carbon -19%
Hydrogen-10%
-nitrogen -3%
-oxygen – 65%
Ions : Electrically charged atoms where # of electrons & protons differ (they've gained or lost electrons from/to other atoms).
Cation (positive ion); anion (negative ion)
Atoms bond with one another to form compounds.
Ionic compound-compound formed using ionic bonds
Molecular compound- compound formed using covalent bonds (O
2
, F
2
)
Atomic Animations:
Atomic Structure
Online Outline of Atoms & Water (McGraw Hill)
Chemical Bonding (Bonding by Analogy: Dog-Bond Bonds)
Ionic vs Covalent Bonding (McGraw Hill)
Ionic Bonds
Chemical Bonds a.
[ Ionic bond ] : atoms donate or receive electrons from other atoms. Form bond based on electrostatic attraction.
Example: sodium chloride (metal +non-metal) b.
[ Covalent bond ] : two atoms share one or more pairs of valence electrons. Can be single double or triple bonds
- Example: diatomic hydrogen
Element Name
Most Frequent Elements Found in Organisms
% Composition by
Body Weight
Importance
Oxygen
Carbon
65%
18.5% a. Used in cellular respiration. b. Component of water. c. Component of organic molecules a. Backbone of organic molecules
Hydrogen
Iron
Element Name
Nitrogen
Calcium
Phosphorus
9.5% a. Electron carrier. b. Component of water. c. Important component of organic molecules.
Other Important Elements Found in Living Organisms
Importance of Element a. Component of all proteins. b. Component of all nucleic acids. a. Component of bones & teeth. b. Muscle contractions. a. Component of nucleic acids (DNA). b. key component of ATP (Adenosine triphosphate- chemical energy molecule ) a.
Component of blood hemoglobin
(carries O2). b.
Used by some bacteria as a source of energy c.
Needed to form chlorophyll
Sodium
Sulfur a. Main positive ion outside cells. b. Important in nerve impulse transmission. c. helps move flagellum a.
Found in amino acids (building blocks of proteins) b.
Can take part is S-S bonds within proteins known as disulphide bridges c.
Source of energy for prokaryotes
Polar Molecules- ex. WATER
electrons shared in covalent bonds may be shared unequally - they may spend more time around one particular atom over another
this is called a polar molecule = molecule in which electrons are not shared equally thus giving one end of the molecule a partially negative charge and the other end a partially positive charge
electronegativity= relative ability of an atom to attract electrons. E.g fluorine is the most electronegative ion (increasing electronegativity as you move up and to the right on the periodic table)
Water (H
2
O)
water is covalent but the electrons are not shared equally
O is more electronegative than H and thus electrons spend more time around O, making it more negative (relative to H) and H is more positive (relative to O)
water is therefore polar
H
∂+
O
∂-
H
∂+
intramolecular bonds = bonds within the molecule (bonds between the atoms that make up each individual molecule)
intermolecular bonds = bonds between molecules
(are generally weaker than intramolecular but cumulatively they can be quite strong)
electrons in the intramolecular bonds of water are not shared equally and thus water is polar
the intermolecular bonds in water are called hydrogen bonds
hydrogen bond (H-bond) = forces of attraction between a hydrogen of one molecule and a highly electronegative element of another molecule (usually F, O, N)
these H-bonds hold water molecules together
Water
Water constitutes two-thirds the mass of most organisms & 75% earth's surface.
Importance of Water to Life
1. Coolant: Has high heat of vaporization, aids body cooling.
2. High specific heat: ( H20 = 1 cal / g / degree C ) - bodies of water stay constant temp.
3. Transport: polarity - dissolves many substances.
4. Habitat: Major component of internal (organism) & external environments
Property
1. Transparency
2. Universal Solvent
3. Cohesion
4. Adhesion
5. High Specific Heat
Capacity
6. High boiling point.
7. Evaporation
8. Density of Ice
Properties of Water
Meaning
Light passes through water.
Importance of Property
Light reaches chloroplasts in cells & aquatic plants.
Many compounds dissolve in water.
Dissolved compounds can be brought to cells (via sap or blood) or move about cell cytoplasm.
Acts as a medium for reactions in the cell.
Water molecules stick together due to H bonds.
Water molecules stick to other molecules.
Small animals/insects like the water strider may walk on water.
Called surface tension
Capillary action. Water pulled to top of trees.
Large amounts of energy are needed to raise temp of water.
-heat absorbed = bonds broken
-heat released = bonds formed
-much of E is used in the disruption or formation of H- bonds and thus E does not affect molecular motion as quickly/dramatically)
Water bodies have stable temperatures.
Body temps can be maintained. Transfer of heat from warm to cool body parts.
Much energy needed to pull water molecules apart.
In nature, water rarely boils so life is spared.
Evaporation (boiling) requires much energy.
Evaporation can cool warm cells.
Ice is less dense than water so it floats.
When water is a solid it expands since it possess a maximum # of
H-bonds which hold water molecules further apart making it less dense
Ice insulates organisms living beneath.
Ice remains on the top of frozen lakes during the winter (liquid is below because it is more dense – allows aquatic organisms to survive winter)
***Specific heat capacity- the amount of energy, E, required to raise the temperature of 1g of a substance by 1 degree Celsius. A measure of how well a substance resists change. The specific heat capacity of water is 4 J/g.
[ See also the Shape of Water ]
Animations of Important Properties of Water:
Solvency of Water (how water dissolves ionic compounds) - Northland University
Hydrogen Bonds - Northland University
Polarity of Water Molecules: Formation of Ice - UC Davis
Chemical Building Blocks of Life
Organic Molecules : Any compound containing C, H (except CO
2
and those containing CO
3
).
Inorganic Molecules: Any compound not containing C (but including CO
2
and those containing CO
3
).
Hydrocarbon : Any compound with a C-H bond.
Monomer : building blocks of larger molecules --------- Polymers : repeating subunits (monomers) bonded together
Macromolecule : these are larger organic compounds produced by organisms. They are required for the various functions of life. They include carbohydrates, lipids, and proteins.
Forming Macromolecules
Dehydration [ Condensation ] : Reactions to build most large organic molecules. (One molecule of water removed from bond area as monomers are linked.)
Hydrolysis : Digestion: Large organic molecules are broken up. Molecule of water added to help remove the monomers.
Biological Macromolecules :
The organic molecules found in living organisms.
[ Proteins ] [ Carbohydrates ] [ Lipids ] [ Nucleic Acids ]
CARBOHYDRATES
Carbos = Sugars = Saccharides: C, H, O in 1:2:1 ratio (roughly CH2O)
These compounds contain carbon, hydrogen, and oxygen
Function-main source of energy. Most easily digested of all macromolecules
Hydrophilic (water loving)
Three categories monosaccharides, disaccharides, polysaccharide o Carbohydrate Monomers = Monosaccharides
Types of Carbohydrates: (scientific names of all carbos end in - ose )
1.
Monosaccharides : simple sugars (The building block of all larger sugars.)
There are 3 main monosaccharides and they are ISOMERS or one another
ISOMERS: molecules which have the same molecular formula but different structural formulas
Examples of Monosaccharides : a) Glucose C
6
H
12
O
6
- Form of simple sugar used by all cells. From grapes & honey. b) Fructose C
6
H
12
O
6
- Fruit sugar c) Galactose C
6
H
12
O
6
- Less sweet. Dairy products & gums.
Another monosaccharide is RIBOSE: C
5
H
12
O
5
Ribose is a 5-carbon sugar (pentose sugar)
It is found in nucleic acids (genetic material)
* see diagram below
Different 6-carbon monosaccharides can be made by switching positions of atoms on the carbon chains.
2. Disaccharides : double sugars –contain two monosaccharides chemically bonded together.
Formed by condensation synthesis (removal of water as the 2 monosaccharides bond)
Breaking apart a disaccharide is a hydrolysis reaction
Examples of Disaccharides: a) Maltose = (malt sugar) glucose + glucose b) [ Sucrose ] (table sugar) = glucose + fructose c) Lactose (milk sugar) = glucose + galactose
2.
Polysaccharides : starches, chains of sugars – a.
3 or more monosaccharides bonded together. b.
Formed by condensation synthesis (removal of water as all the monosaccharides bond) c.
Provide energy storage and carbohydrate storage for organisms
Examples of Polysaccharides: a) Amylose : simple plant starch (energy storage) b) Pectins : branched plant starch (gelling agent) c) Glycogen : branched glucose a.
animal energy storage b.
secondary long-term energy storage. Stored in the liver and muscles) d) Cellulose : component of plant cell walls, indigestible by most organisms, human dietary fiber
Importance of Carbohydrates: a) Glucose - key metabolic fuel (energy source) of all cells. b) Animal Starch ( Glycogen )- long term energy storage for animal cells (stores the glucose molecules in a form not easily used!). c) Plant Starch ( Amylose ) - long term energy storage for plant cells (stores the glucose molecules in a form that is not easily used!) d) Cellulose - Structural polysaccharide of cell walls. e) Chitin - Structural polysaccharide of exoskeletons of insects and crustaceans.
Recognize and Learn How to Draw from Memory the Following Carbohydrates:
---------------------
Ribose is the 5 carbon sugar found in RNA (ribonucleic acid).