3rd nine week chemistry syllabus
advertisement
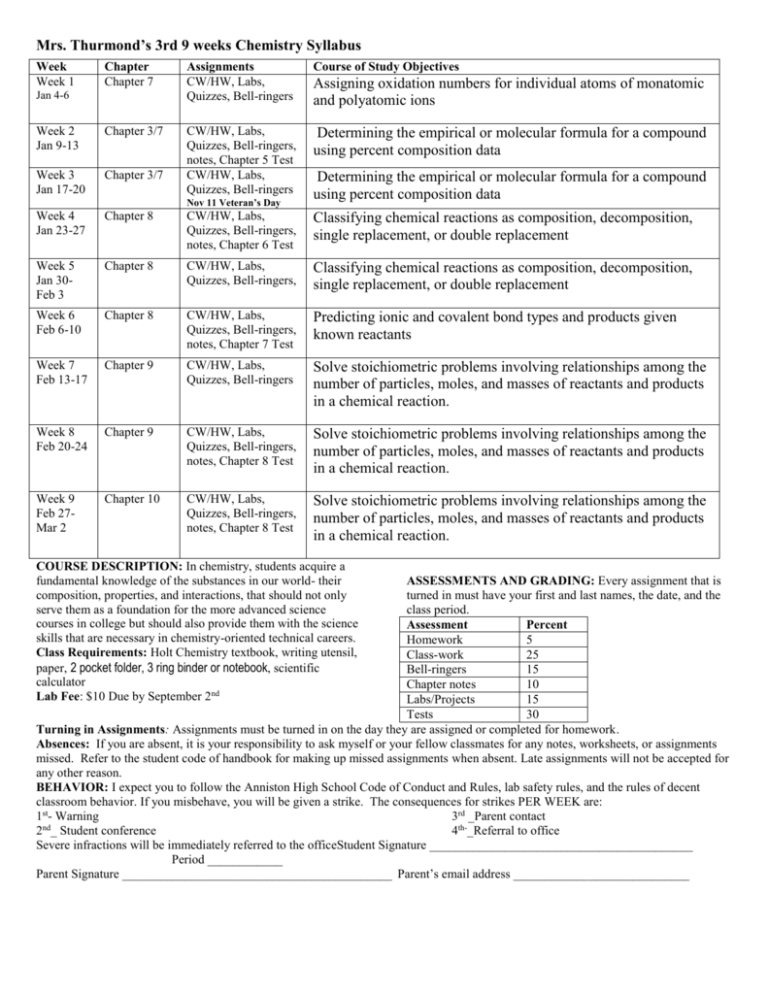
Mrs. Thurmond’s 3rd 9 weeks Chemistry Syllabus Week Week 1 Chapter Chapter 7 Assignments CW/HW, Labs, Quizzes, Bell-ringers Course of Study Objectives Week 2 Jan 9-13 Chapter 3/7 Determining the empirical or molecular formula for a compound using percent composition data Week 3 Jan 17-20 Chapter 3/7 CW/HW, Labs, Quizzes, Bell-ringers, notes, Chapter 5 Test CW/HW, Labs, Quizzes, Bell-ringers Week 4 Jan 23-27 Chapter 8 CW/HW, Labs, Quizzes, Bell-ringers, notes, Chapter 6 Test Classifying chemical reactions as composition, decomposition, single replacement, or double replacement Week 5 Jan 30Feb 3 Chapter 8 CW/HW, Labs, Quizzes, Bell-ringers, Classifying chemical reactions as composition, decomposition, single replacement, or double replacement Week 6 Feb 6-10 Chapter 8 CW/HW, Labs, Quizzes, Bell-ringers, notes, Chapter 7 Test Predicting ionic and covalent bond types and products given known reactants Week 7 Feb 13-17 Chapter 9 CW/HW, Labs, Quizzes, Bell-ringers Solve stoichiometric problems involving relationships among the number of particles, moles, and masses of reactants and products in a chemical reaction. Week 8 Feb 20-24 Chapter 9 CW/HW, Labs, Quizzes, Bell-ringers, notes, Chapter 8 Test Solve stoichiometric problems involving relationships among the number of particles, moles, and masses of reactants and products in a chemical reaction. Week 9 Feb 27Mar 2 Chapter 10 CW/HW, Labs, Quizzes, Bell-ringers, notes, Chapter 8 Test Solve stoichiometric problems involving relationships among the number of particles, moles, and masses of reactants and products in a chemical reaction. Jan 4-6 Nov 11 Veteran’s Day Assigning oxidation numbers for individual atoms of monatomic and polyatomic ions Determining the empirical or molecular formula for a compound using percent composition data COURSE DESCRIPTION: In chemistry, students acquire a fundamental knowledge of the substances in our world- their composition, properties, and interactions, that should not only serve them as a foundation for the more advanced science courses in college but should also provide them with the science skills that are necessary in chemistry-oriented technical careers. Class Requirements: Holt Chemistry textbook, writing utensil, paper, 2 pocket folder, 3 ring binder or notebook, scientific calculator Lab Fee: $10 Due by September 2nd ASSESSMENTS AND GRADING: Every assignment that is turned in must have your first and last names, the date, and the class period. Assessment Percent Homework 5 Class-work 25 Bell-ringers 15 Chapter notes 10 Labs/Projects 15 Tests 30 Turning in Assignments: Assignments must be turned in on the day they are assigned or completed for homework. Absences: If you are absent, it is your responsibility to ask myself or your fellow classmates for any notes, worksheets, or assignments missed. Refer to the student code of handbook for making up missed assignments when absent. Late assignments will not be accepted for any other reason. BEHAVIOR: I expect you to follow the Anniston High School Code of Conduct and Rules, lab safety rules, and the rules of decent classroom behavior. If you misbehave, you will be given a strike. The consequences for strikes PER WEEK are: 1st- Warning 3rd _Parent contact nd 2 _ Student conference 4th-_Referral to office Severe infractions will be immediately referred to the officeStudent Signature __________________________________________ Period ____________ Parent Signature ___________________________________________ Parent’s email address ____________________________







