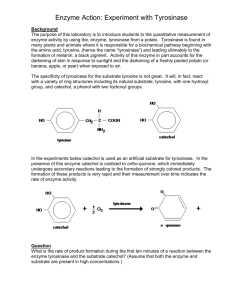
3. Biochemical properties and distribution of
advertisement

1 Bacterial tyrosinases and their applications 2 3 Greta Faccioa,b, Kristiina Kruusb, Markku Saloheimob, Linda Thöny-Meyera 4 5 6 a Empa, Swiss Federal Laboratories for Materials Science and Technology - Laboratory for Biomaterials, Lerchenfeldstrasse 5, CH-9014 St. Gallen, Switzerland b VTT Technical Research Centre of Finland, P.O. Box 1000, FI-02044 VTT, 02044 Espoo, Finland 7 8 9 Greta.Faccio@empa.ch - Kristiina.Kruus@vtt.fi - Markku.Saloheimo@vtt.fi – Linda.Thoeny@empa.ch 10 11 Corresponding author 12 Linda Thöny-Meyer 13 Empa, Swiss Federal Laboratories for Material Sciences and Technology 14 Laboratory for Biomaterials, Lerchenfeldstrasse 5 15 CH-9014 St. Gallen, Switzerland 16 Tel. +41 58 765 7792 1 17 Abstract 18 19 20 21 22 23 24 25 26 27 28 29 30 Tyrosinases with different physico-chemical properties have been identified from various bacterial phyla such as Actinobacteria and Proteobacteria and their production is often inducible by environmental stresses. Tyrosinases are enzymes catalysing the oxidation of mono- and di-phenolic compounds to corresponding quinones with the concomitant reduction of molecular oxygen to water. Since the quinone produced can further react non-enzymatically with other nucleophiles, e.g. amino groups, many tyrosinases have a recorded cross-linking activity on proteins. Various bacterial tyrosinases oxidise tyrosine, catechol, L/DDOPA, caffeic acid and polyphenolic substrates such as catechins. This substrate specificity has been exploited to engineer biosensors able to detect even minimal amounts of different phenolic compounds. The physiological role of tyrosinases in the biosynthesis of melanins has been used for the production of coloured and dyeing agents. Moreover, the cross-linking activity of tyrosinases has found application in food processing and in the functionalization of materials. Numerous tyrosinases with varying substrate specificities and stability features have been isolated from bacteria and they can constitute valuable alternatives to the well-studied tyrosinase from common mushroom. 31 32 Keywords 33 Tyrosinase, biosynthesis, biosensors, bioremediation, food, dyeing 34 Abbreviations 35 36 37 ABTS, 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid); COD, chemical oxygen demand; DOPA, 3,4-dihydroxyphenylalanine; SDS PAGE, sodium dodecyl sulphate polyacrylamide gel electrophoresis; TYR, tyrosinase. 38 2 39 40 41 42 43 44 45 46 47 48 1. Introduction 49 50 51 52 53 54 Tyrosinases are copper-dependent enzymes. They catalyse the ortho-hydroxylation of monophenols such as tyrosine and the subsequent oxidation to quinones (Figure 1). Tyrosinases comprise the activity of catechol oxidases (EC. 1.10.3.1), a family of structurally similar enzymes whose activity is limited to diphenolic substrates. However, the substrate specificity of tyrosinases and catechol oxidases can overlap with the one of laccases (EC 1.10.3.2) which are structurally different enzymes lacking monophenol hydroxylase activity, and these enzymes are sometimes grouped under the name ‘polyphenol oxidase’. 55 56 57 58 59 60 61 62 To date, three three-dimensional structures from tyrosinase have been published. In 2006, the first crystal structure of a tyrosinase from the bacterium Streptomyces castaneoglobisporus was solved [5]. In 2011, the three-dimensional structure of the bacterial tyrosinase from Bacillus megaterium as well as that of the fungal enzyme from Agaricus bisporus were published [6, 7]. Additionally, the three-dimensional structure of two structurally related enyzmes, the catechol oxidases from sweet potato (Ipomoea batata) and the polyphenol oxidase from grapes (Vitis vinifera) are available [8, 9]. Recent sequence analyses revealed a similarity between bacterial and fungal polyphenol oxidases (tyrosinases) which carry features that are not present in the corresponding enzymes from plants [10]. 63 64 65 66 67 68 69 70 71 The application of tyrosinases to different fields, ranging from food to materials, relies on the ability of these enzymes to oxidise phenolic groups from both small molecules such as tyrosine to polymeric substrates such as proteins, thus enabling polymer cross-linking. Although many bacterial tyrosinases have been characterised to some extent, the information about them, e.g. substrate specificity and stability features, is neither easily accessible nor has it been clearly summarised. Bacterial tyrosinases have been the subject of two previous review articles dealing with the structural features [11] and the molecular properties [12] of this class of enzymes. A review focusing on tyrosinases from streptomycetes has been published recently [13]. The present review provides a more comprehensive overview on the biochemical properties of the reported bacterial tyrosinases and on their various applications in different fields. 72 73 74 75 76 77 78 79 80 2. Reaction and structural features of tyrosinase Tyrosinases oxidise phenolic hydroxyl groups of small molecules or large polymeric substrates such as proteins. Tyrosinases catalyse first the ortho-hydroxylation of the phenolic substrate and second its subsequent oxidation to quinone (Figure 1) with the concomitant reduction of oxygen to water. The reaction is chromogenic as the quinones produced can undergo further non-enzymatic polymerisation to form black eu-melanins and, when reacting with thiol groups, brownish pheo-melanins [14]. This process can be inhibited by antioxidants such as ascorbic acid, for example to prevent the browning reaction in food preparations [15]. Tyrosinase activity is generally measured by either determining the consumption of oxygen during the reaction or spectrophotometrically by following the increase of absorbance at 475 nm due The ability of bacteria to produce melanins has long been known. Tyrosinase (EC 1.14.18.1), the key enzyme initiating the biosynthetic pathway, has been characterised in many species. Genes coding for proteins that carry the characteristic tyrosinase domain have been identified in many of the bacterial genomes sequenced to date. Similarly, tyrosinases are present in fungi, plants and animals. The synthesis of microbial melanin can also involve other enzymes than tyrosinase such as laccases, polyketide synthases, phydroxyphenylpyruvate oxidase and 4-hydroxyphenylacetic acid hydroxylase [1]. Bacterial melanin plays a protective role in different ways: it protects DNA from the damages of UV radiation and reactive oxygen species [2], it is able to bind toxic heavy metals [3] and to interact with DNA, possibly slowing down the metabolism [4]. Tyrosinases are thus important for the survival of the organisms. 3 81 82 to dopachrome formation. The cross-linking activity of tyrosinase on proteins is usually analysed by SDS PAGE, size-exclusion chromatography, UV spectroscopy or mass spectrometry [16]. 83 84 85 86 87 88 89 90 The active site of tyrosinases interacts with both the phenolic substrates and the co-substrate oxygen and it alternates among three different oxidation states. When in the oxy state, tyrosinase binds oxygen and is able to catalyse the hydroxylation of monophenols to diphenols, thus changing into the met form. The met form of tyrosinase is responsible for the oxidation of diphenols to quinones and the reaction turns the enzyme into the deoxy form that, upon binding molecular oxygen, returns to the oxy form. The met form is the resting state of the enzyme and it has been calculated that up to 85% of the enzyme is in this state when in solution [17, 18]. The inability of most of the enzymes in an enzyme population to act on monophenols explains why a significant lag phase is detected in the activity when monophenols are the substrate of the reaction. 91 92 93 94 95 96 97 98 99 100 101 102 103 104 105 106 107 Bacterial tyrosinases have been divided in five types according to the organisation of domains and the possible requirement of a caddie protein for enzyme activity [12]. The necessity of a secondary helper protein (caddie protein) for secretion, correct folding, assembly of the copper atoms and activity of the enzyme is common to tyrosinases of type I, e.g. the enzyme from S. castaneoglobisporus and S. antibioticus [19, 20]. Type II tyrosinases are small, monomeric enzymes containing only the catalytic domain, which do not require additional helper proteins and are possibly secreted. An example is the tyrosinase from B. megaterium [6]. Type III tyrosinases are represented by the enzyme from Verrucomicrobium spinosum. Like the fungal tyrosinases it carries a C-terminal domain whose removal led to about 100-fold higher activity [21]. This supports the theory that the role of the C-terminal extension in plant and fungal tyrosinases is to keep the enzyme in an inactive form inside the cell [22-24]. Among the smallest bacterial tyrosinases reported (Type IV) are the ones produced by Streptomyces nigrifaciens (18 kDa) and Bacillus thuringiensis (14 kDa) [25, 26]. However, it is debated whether these proteins are true tyrosinases [12]. Type V tyrosinases include enzymes that do not carry the sequence features of tyrosinases but show features typical of laccase and have only marginal activity on tyrosine. For example, a membrane-bound tyrosinase active on the typical laccase substrate ABTS (NCBI ID: AAF75831.2) has been isolated from Marinomonas mediterranea. A tyrosinase with a classical substrate specificity that is activated by SDS (NCBI ID: AAV49996.1) has also been reported from the same organism [27]. 108 109 110 111 112 113 114 115 116 117 118 Similar to catechol oxidases and the oxygen carrying haemocyanins, tyrosinases are type-3 copper proteins, containing two copper atoms in the active site. The absorbance spectrum of oxy-tyrosinases has a characteristic maximum in the UV region (330-345 nm). As reported for the structurally similar catechol oxidases, a fluorescence intensity maximum at 330 nm upon excitation at 280 nm is also detected [28, 29]. Copper is essential for the catalytic activity of tyrosinases. The crystal structure of these enzymes has demonstrated the presence of two copper ions in the catalytic core (Table 1). In all tyrosinases of different origins and in the haemocyanins each of the copper ions is coordinated by three histidine residues that are found in a characteristic pattern in the primary structure (Figure 2). In the tyrosinase from Streptomyces glaucescens, for example, the key role of histidines at position 37, 53, 62, 189, 193 and 215 in the coordination of copper, and thus in catalytic activity, was confirmed by the decrease of activity upon their substitution with other amino acids [30, 31]. 119 120 121 122 123 124 Various additional residues have been identified to have a function in fungal and bacterial tyrosinases, either being essential for or modulating tyrosinase activity. Sequence analysis and various mutagenesis studies have been performed in order to identify the residues necessary for the activity of the enzyme. In tyrosinase sequences from plants and fungi, the N-terminal signal peptide, when present, is followed by a conserved arginine residue that marks the beginning of the central catalytic domain and that forms a pi-cation interaction with a conserved C-terminal Y/FXY tyrosine motif, where X is any amino acid [32]. These 4 125 126 127 128 129 130 131 132 133 134 135 136 137 138 139 140 141 142 143 144 145 146 147 148 149 150 residues are conserved also in bacterial tyrosinases (Supplementary file 1). Substitution of the N-terminal conserved arginine (R40) has been reported to abolish the production of tyrosinase from V. spinosum [21]. Two single-amino acid substitutions have been reported to improve the catalytic activity of the tyrosinase from Rhizobium etli CFN42. The independent replacement of proline at position 334 and of aspartic acid at position 535 (Supplementary file 1) with a smaller residue such as serine (P334S) or glycine (D535G), respectively, led to a significant enhancement of the catalytic activity and melanin formation [33-35]. In the tyrosinase from B. megaterium, a single substitution of arginine by histidine within the copper B binding region (R209H) has been sufficient for a 1.7-fold improvement of the activity towards tyrosine (monophenolase) and for a 1.5-fold reduction of activity on L-DOPA (diphenolase), whereby the overall protein stability was not affected [36]. The crystal structure of the tyrosinase from B. megaterium showed that this arginine is positioned at the entrance of the active site in a flexible position and plays a role in the docking of the substrate [6]. However, the conservative substitution of the corresponding residue asparagine 190 to glutamine (N190Q) in S. glaucescens tyrosinase abolished the catalytic activity, indicating that this residue was possibly involved in hydrogen bonding at the active site [30]. Moreover, the conservative substitution of the residue aspartic acid 209 (D209E) has been reported to stabilise the oxy-form of the same enzyme [37]. To our knowledge, no study has investigated the role of the oxygen binding motif PYWDW [38] with regards to the affinity for oxygen in tyrosinase. The affinity for the co-substrate oxygen has been evaluated for the tyrosinase from Streptomyces antibioticus that carries the PYWDW motif. It was found that this enzyme had a three-fold lower dissociation constant (kD) for oxygen than the A. bisporus tyrosinase [39, 40] that carries a PFWDW motif, i.e. 16.5 μM compared to 46.6 μM. The analysis of the characterised bacterial tyrosinases evidenced the presence of functionally active variants of this motif (Supplementary file 1 and 2), e.g. PYWNY in the tyrosinase from M. mediterranea, PFWDW in tyrosinase from R. etli, PYWEW in the tyrosinase from B. megaterium, PYWRF and PYWNW in the tyrosinases from Ralstonia solanacearum. Mutational studies have also addressed the interaction of tyrosinases from streptomycetes and their caddie protein. In S. antibioticus, the two histidine residues at positions 102 and 117 of the caddie protein MelC1 have been found to be crucial for the biosynthesis of active tyrosinase [41]. 151 152 153 154 155 156 157 158 The available crystal structures of bacterial tyrosinases and their mutant forms have been obtained from Gram-positive S. castaneoglobisporus and B. megaterium (Table 1). While the B. megaterium tyrosinase formed crystals containing only the enzyme, the S. castaneoglobisporus tyrosinase required the presence of a second protein, referred to as caddie protein, to stabilise its structure [4]. Moreover, the structure of the Streptomyces tyrosinase has been solved in different states of oxidation. Aiming at understanding the interaction between tyrosinase and caddie protein, tyrosinase has been crystallised in the presence of mutant forms of the caddie protein (Table 1). Likewise, the fungal tyrosinase from A. bisporus was crystallised as a tetramer in a complex with a second protein, a lectin-like protein [7]. 159 160 161 162 163 164 165 166 167 168 169 Both intracellular and secreted bacterial tyrosinases have been isolated and characterised. For example, the tyrosinases from Streptomyces nigrifaciens, Bacillus thuringiensis, M. mediterranea, R. solanacearum and Thermomicrobium roseum were isolated from cell biomass and the ones from S. antibioticus, S. glaucescens, S. castaneoglobisporus, Streptomyces albus, B. megaterium, Sinorhizobium meliloti, Aeromonas media, R. etli and V. spinosum were either isolated from the culture medium or predicted to be secreted [19, 21, 25, 26,42-51]. The twin-arginine signal peptide is often found in cofactor-binding oxidoreductases that undergo complete folding in the cytoplasm prior to secretion to the periplasmic or extracellular space. Twin-arginine type signal peptides [52] could be identified in the N-terminal region of tyrosinases from R. solanacearum (34-amino acid long) and V. spinosum (33-amino acid long). A more detailed analysis of the sequence retrieved for the tyrosinase from R. etli and the alignment with the other sequences of tyrosinases (Supplementary File 1) suggests the possibility of incorrect open reading frame prediction. The true N5 170 171 172 173 terminal methionine may be M112 (underlined in Supplementary file 1) as it aligns with the initial residue of the tyrosinase from R. solanacearum (number 15 in Supplementary file 1) and is followed by a predicted twin-arginine signal peptide of 31 amino acids [51]. Thus, we suggest that these proteins purified from the cell biomass but carrying a signal peptide for secretion are localised in the periplasm. 174 175 176 177 178 179 180 181 182 183 184 185 186 187 188 189 190 191 192 193 Tyrosinases, also from bacteria, and their caddie proteins generally lack conserved cysteine residues (for comments see [11, 12]). The paucity of cysteine residues, and thus disulphide bonds, allowed, however, the isolation of tyrosinases with significant thermal stability, e.g. the enzyme from B. megaterium had an optimum temperature of 50°C [48]. A single cysteine residue is conserved in proximity of the second histidine residue of the copper A binding motif in the characterised tyrosinases from M. mediterranea, R. solanacearum, S. meliloti, R. etli and V. spinosum (Supplementary file 1). A cysteine residue at this position has been found to be covalently bound to a histidine residue two positions forward in, for example, the fungal tyrosinase from Neurospora crassa [53], the plant catechol oxidase from I. batata [8] and haemocyanins from the snail Helix pomatia [54]. The function of this unusual cysteine-histidine bond is not established, but it could confer structural rigidity to the copper-binding region and affect the redox potential [8]. Replacement of this cysteine residue (C84) with serine abolished the production of the tyrosinase from V. spinosum [21]. Type-3 copper proteins carrying six conserved cysteines (forming three in silico predicted disulphide bonds) and characterised by significant thermal stability have been reported in fungi [26]. No mutagenesis study has addressed a possible improvement of the thermal stability of bacterial tyrosinases by introducing disulphide bonds. However, in silico analysis revealed the possible presence of one disulphide bond in the tyrosinases from R. solanacearum and S. meliloti and two in the enzymes from M. mediterranea and R. etli (Dianna software, http://clavius.bc.edu/~clotelab/DiANNA). The tyrosinase from S. castaneoglobisporus and the one from B. megaterium share approximately 30% sequence similarity with a catechol oxidase from Aspergillus oryzae that showed a melting temperature above 70°C and a half-life of 20 hours when incubated at 50°C [29]. 194 195 196 197 198 199 It should be noted that the tyrosinase from A. media exhibits different sequence features when compared to the other enzymes. The sequence alignment with bacterial tyrosinases shows that none of the typical signature motifs (copper A and B regions, oxygen binding motif and tyrosine motif) are present (see Supplementary file 1). Moreover, this enzyme has a predicted 23-amino acid long signal peptide [50] and shows strong sequence similarity to bacterial periplasmic proteins that are responsible for the uptake of peptides and involved in nutrition and sensing of the environment [55]. 200 201 202 203 204 205 3. Biochemical properties and distribution of bacterial tyrosinases Although the ability of bacteria to synthesise melanin has been reported for various species (the latest example being the proteobacterium Brevundimonas sp. SGJ [56]), the information concerning the characterisation of purified bacterial tyrosinase enzymes is limited and not easily available. Tyrosinases with different biochemical properties and cellular localisation have been identified from organisms belonging to different bacterial phyla (Figure 3), particularly from streptomycetes. 206 207 208 209 210 211 212 Many bacterial genomes carry more than one operon containing tyrosinase-coding genes. The genome analysis of the Gram-positive streptomycetes, for example, revealed two operons melC and melD, responsible for the production of two tyrosinases, MelC2 and MelD2, respectively, with quite different properties [57]. The melD operon was identified in all the genomes analysed, while the melC operon was only present in the melanin-producing Streptomyces strains. Tyrosinase MelC2 is secreted and has activity on a wide range of substrates, whereas MelD2 is intracellular (membrane-associated) and has a narrower substrate specificity, i.e. it is not active on ortho-aminophenol and caffeic acid [57]. Melanin production is 6 213 214 215 216 217 218 219 220 221 222 223 associated with the presence of the operon containing the tyrosinase MelC2, and albino mutants have been found to harbour only the melD operon. MelC2 has been shown to promote catechol uptake by the cells, by oxidising them to more hydrophobic quinones. MelD2 has been suggested to play a protective role against the toxic oxygen-reactive species that can be spontaneously produced from phenolic compounds in the cell [57]. Two genes coding for tyrosinase have also been identified in the genome of the Gram-negative plant pathogen R. solanacearum (NCBI ID: NP_518458 and NP_519622). Although both corresponding intracellular proteins carried the sequence features typical of tyrosinases, their biochemical characterisation revealed that the former had a significant preference for monophenols and the latter for diphenols, like a catechol oxidase [43]. In cases when the tyrosinase requires the co-expression of a caddie protein for its secretion and assembly such as the tyrosinase from S. castaneoglobisporus [20] and from M. mediterranea [58], both genes are located within the same operon. 224 225 226 227 228 229 230 231 232 233 234 235 236 237 238 239 240 241 242 The available information suggests that in general bacterial tyrosinases have a pH optimum around 7.5 and an optimal working temperature around 40°C (Figure 3). Bacterial tyrosinases are generally monomeric enzymes with a molecular mass between 20 and 60 kDa (Figure 3). A dimeric bacterial tyrosinase has been isolated from the highly thermophilic bacterium T. roseum (Figure 3, number 21) [44]. Robust bacterial tyrosinases with physico-chemical characteristics ideal for industrial applications have been identified from various strains. For example, a 31 kDa tyrosinase with a maximum activity at 50°C was described from B. megaterium and tyrosinases with enhanced activity in the presence of organic solvents have been isolated from B. megaterium and Streptomyces REN-21 [48, 59] and a tyrosinase with optimal working temperature of 70°C and pH 9.5 from T. roseum [44]. Typical substrates for tyrosinases are monophenols such as tyrosine and modified tyrosine, phenol and coumaric acid and diphenols such as the model substrate LDOPA or caffeic acid; polyphenols of different sizes including pyrogallol and catechins can also be substrates for tyrosinases (Table 2). The substrate specificity of tyrosinase has been altered not only by mutagenesis [37] but also by changing the reaction conditions. For example, the B. megaterium tyrosinases showed a 5-fold higher monophenolase/diphenolase activity in the presence of ionic liquids [60]. The oxidation of protein-bound tyrosines to quinone-like structures by tyrosinase promotes their reaction with other tyrosine, cysteine or histidine residues of the same or a different polypeptide chain thus forming intraand inter-molecular cross-links [61]. Recently, the cross-linking activity of the V. spinosum tyrosinase has been demonstrated on tyrosine-containing model proteins of different sizes, e.g. cytochrome c (11.7 kDa) or lipase B from Candida antarctica (33.4 kDa) [62]. 243 244 245 246 247 248 249 250 251 252 253 254 255 256 4. Production of bacterial tyrosinases The production of tyrosinase and the associated synthesis of melanin are reported to be naturally induced by exogenous stresses, such as heat and hyperosmotic stress, and by specific compounds such as tyrosine as well as in the presence of copper, the essential metal cofactor (Table 3). In S. antibioticus, the induction by L-methionine promotes fast secretion of the enzyme without intracellular accumulation, and cultivation in the absence of copper resulted in the production of the enzyme in the apo-form [19]. Studies in Streptomyces species revealed that induction is regulated at both the transcriptional and translational level [19, 63]. Similarly, wounding and methyl jasmonate have been identified as inducers of tyrosinase production in plants [64]. The expression of fungal tyrosinases is known to be triggered by bacterial infection [65], exposure to light [66], culture medium composition [67] and the presence of copper [68]. By contrast, specific compounds have a repressive effect on the production of tyrosinase. In bacteria, ammonium has been identified as a repressor for the production of tyrosinase from Streptomyces michiganensis [69, 70] and in fungi, amino acids and analogues such as D-tyrosine act as repressors in the production of a tyrosinase from Neurospora [71]. 7 257 258 259 260 261 262 263 264 265 266 267 268 269 Currently, the tyrosinase from the fungus A. bisporus is still the only commercialised tyrosinase [72]. The production of bacterial tyrosinases has not only taken advantage of the natural production, but it has also been carried out heterologously in host strains. Scarce data are available about the production of bacterial tyrosinases that is often identified only by the production of melanin. S. albus is a natural tyrosinase producer and 0.2 mg/l of purified tyrosinase could be isolated from the culture medium [47]. A production level of 20 mg of purified protein per liter of culture was achieved when the operon containing the gene coding for the tyrosinase from S. antibioticus was overexpressed in the native host [73]. Upon overexpression in E. coli, production levels of 86 mg/l of purified tyrosinase from B. megaterium [48] and approximately 20 mg/l of tyrosinase from V. spinosum [21] were achieved. The tyrosinase from Streptomyces REN-21 showed activity on tyrosine-containing peptides and was recombinantly produced in E. coli with a production level of 54 mg/l [74]. Among others, the tyrosinases from Pseudomonas maltophila and R. etli have also been recombinantly produced in E. coli, but there is no information on the production levels obtained [32, 51, 75, 76]. 270 5. Applications of tyrosinases 271 272 273 274 275 276 Most suggested applications of tyrosinases have been tested with the commercially available mushroom enzyme. However, considering the similar reactivities, these applications are conceivable also for bacterial tyrosinases. The ability of tyrosinase to act on catechol-like substrates was the subject of patenting in 1970 [77] and the cross-linking activity of tyrosinases on peptides was patented in 2003 [78, 79]. In some cases, the enzyme was not used in an isolated form, and natural tyrosinase-producing strains were employed in the process, e.g. in bioremediation [80]. 277 278 279 280 281 282 Applications of tyrosinase rely on the ability of tyrosinases to oxidise both small phenolic molecules and protein-associated phenolic groups, i.e. the side chain of the amino acid tyrosine [16, 77, 78]. Due to the various potential applications of tyrosinases (Figure 4) not only have fungal enzymes been subjects of patent applications but also bacterial enzymes such as the tyrosinases from V. spinosum, R. etli, S. antibioticus and Pseudomonas sp. DSM13540 [46, 81, 82, 83]. An overview of representative applications of tyrosinases in different fields is given in Table 4. 283 284 285 286 287 288 289 290 291 Various aspects of tyrosinase activity are desirable. In molecular biology the chromogenic reaction catalysed by the tyrosinase from S. glaucescens has been used as a reporter for gene expression [84], and the enzyme from R. etli has served as a tool to detect bacterial strains producing L-tyrosine [34, 35]. The ability of tyrosinases to oxidise small phenolic molecules can be exploited for the removal of these substrate compounds from environmentally polluted samples (bioremediation), for the synthesis of secondary compounds and for initiating the chromogenic melanin-synthesis process (biocatalysis and dyes production). The ability of tyrosinases to act on larger molecules such as peptides and proteins containing tyrosine (crosslinking activity) has been exploited, for example, to prepare adhesive solutions and to modify the protein structure of food [85, 86]. 292 293 294 295 296 297 298 Tyrosinase has been used not only in free form but also in an immobilised [87] and cross-linked aggregated form [88]. Immobilization increased its stability and facilitated reusability. A review focusing on the applications of immobilised tyrosinase was published in 2012 [89]. A similar effect has been reported for tyrosinase immobilised on solid supports such as silica [90], magnetic beads [91] and embedded in selfadhesive layers made of plant-derived agarose and guar gum [92]. In addition, the immobilisation of tyrosinase on clay coated with hydroxyl-aluminium not only increased the specific activity of the tyrosinase but also its temperature stability [93]. Aiming at L-DOPA production, mushroom tyrosinase has also been 8 299 300 immobilised using glutarhaldehyde on chemically modified nylon [94], on sodium aluminosilicate and on calcium aluminosilicate, and two modified forms of zeolite [95]. 301 302 303 304 305 306 307 308 5.1 Tyrosinase and the production of dyes Considering the physiological role of tyrosinase in the synthesis of melanin from tyrosine, the enzyme has been applied to the synthesis of dyeing and colouring solutions. For example, tyrosinase from R. etli has also been used for melanin production in E. coli, and production levels of melanin reached 6 g/l [76]. As early as 1947, tyrosinase was proposed for the dyeing of animal fibres [96]. More recently, a colouring composition containing the enzyme from A. bisporus and L-DOPA has been proposed for colouring hair [97]. However, tyrosinase from mushroom (possibly A. bisporus) has also been tested for colouring goat hair, but little effect was noted [98]. 309 310 311 312 313 314 315 316 5.2 Tyrosinase and biosensors Various biosensors have been developed using mushroom and bacterial tyrosinases to monitor even subpicomolar amounts of phenolic compounds in a sample [99-101]. Concerning application to food products, a tyrosinase/beta-galactosidase-coupled reaction has been exploited to develop a disposable biosensor able to quantify the amount of toxic cyanogenic glycosides from foods such as the kernels of apricot, peach and cherry [102]. Mushroom tyrosinase has also been applied for the determination of the phenolic content of fruit juices, tea, infusions and jams [103, 104]. The development of biosensors for phenol detection has been reviewed recently [105]. 317 318 319 320 321 322 5.3 Tyrosinase for biosynthesis and medical applications The substrate specificity of most tyrosinases is generally wide and substrates include various mono-, di- and poly-phenolic compounds. This qualified tyrosinase for the production of ortho-diphenols, also-called substituted catechols that are essential intermediates in the synthesis of pharmaceuticals, plastics, antioxidants and agrochemicals. For example, the use of tyrosinase in combination with toluene-4monooxygenase has improved the production of 4-fluorcatechol [106]. 323 324 325 326 327 328 329 330 The first product of the tyrosinase reaction with tyrosine, L-DOPA, has a high economical value because it is the main drug for the treatment of Parkinson’s disease and a forecast of predicted sales for 699 MUS$ in 2019 [107]. L-DOPA is currently produced at industrial scale by chemical synthesis, and many studies have been targeted at providing an alternative enzyme-based process using free or immobilized tyrosinase [91, 94, 95, 108]; however, the approach using the enzyme tyrosine-phenol lyase that does not catalyse the further oxidation of L-DOPA might be more promising [109-110]. The di-phenolic product of the reaction with tyrosinase is able to react with cysteine, and tyrosinase can thus be used to produce catecholamines such as cysteinyl-DOPAs [111]. 331 332 333 334 335 Mushroom tyrosinase has been applied to the synthesis of natural compounds with estrogenic activity such as coumestan and derivatives that are generally isolated from plant material [112]. The same enzyme has also been used for the production of the antioxidant hydroxytyrosol in the presence of ascorbic acid (vitamin C), and the final reaction mixture could be used directly as food additive [113, 114]. In principle, bacterial tyrosinases should also be able to catalyse all these reactions and might allow whole cell biotransformations. 336 337 338 339 Medical applications of tyrosinase include also the production of melanins as natural antibacterial compounds for the treatment of wounds, i.e. the local application of melanin precursor and tyrosinase in the form of a cream or ointment [115]. The involvement of tyrosinases in the nervous system is not clear. In an old US patent it was claimed that melanin plays a protective role in the nervous system [116]. Tyrosinase 9 340 341 342 343 has also been tested for the treatment of neuronal diseases; it has been reported to enhance dopamine toxicity, but a genetic association with Parkinson’s disease has not been described [117]. Furthermore, tyrosinase has been suggested as a reporter enzyme for the measurement of cholesterol levels in a skin test [118]. 344 345 346 347 348 349 350 351 352 353 354 355 356 357 5.4 Tyrosinase in bioremediation of wastewaters Bacterial tyrosinases have been tested in the detoxification of wastewaters by removal of phenolic compounds and decolourisation. The tyrosinase from S. antibioticus, for example, had activity on industrial pollutants such as 3- and 4-chlorophenols [119] and 3- and 4-fluorophenols [120]. The application of bacterial tyrosinase to the treatment of contaminated wastewaters has recently been reviewed [121, 122] and can be done either with tyrosinase-producing strains [80] or with the enzyme in an immobilized form as protagonist [123]. For example, tyrosinase (mushroom) has been reported to proceed in a precise order of efficiency in the oxidation of phenolic compounds from wastewaters, e.g. favouring catechol, to p-cresol, pchlorophenol, phenol and p-methoxyphenol [124]. Similar effects have been observed after immobilization of the mushroom tyrosinase on chitosan beads, which allowed the removal of chlorophenols and alkylsubstituted phenols from artificial wastewaters [125]. Bacterial tyrosinase may also have a potential application in decolourization of effluents, as it has been reported for two different species of Basidiomycetes, e.g. Trichosporon akiyoshidainum and Trichosporon beigelii NCIM-3326 [126, 127] to be involved in the degradation of different coloured dye molecules commonly used by the textile industry. 358 359 360 361 362 363 5.5 Tyrosinase and materials The cross-linking activity of tyrosinase has been used to functionalize materials such as chitosan by crosslinking particular enzymes of interest to this biomaterial. Target enzymes can be substrates for the crosslinking reaction in their native form if they have surface exposed tyrosyl groups. For example, organophosphorus hydrolase, chloramphenicol acetyltransferase and cytochrome c have been shown to retain activity upon coupling to chitosan [128]. 364 365 366 367 368 369 370 371 372 373 374 The reactivity of the oxidised tyrosyl group, a quinone produced by tyrosinase (A. bisporus), with free amino groups of a polymer has been exploited to functionalise chitosan with the tyrosine-containing peptide YGG(KVSALKE)5GGC (Kcoil) that is able to recruit proteins carrying the partner peptide (EVSALEK)5 (Ecoil) via coiled-coil interactions [129]. In a similar manner, mushroom tyrosinase has been used to form covalent protein-polysaccharide bioconjugates by oxidising the tyrosine residues of silk proteins sericin and sericin-derived peptides such that they subsequently react with the free amino groups of chitosan [130, 131]. The tyrosinase-catalysed binding of silk proteins to chitosan reduced the particle size of the material, made it more compact, increased its thermal stability and reduced its adhesiveness, making it suitable for medical applications [130, 131]. Mushroom tyrosinase has also been suggested for the site-directed attachment of tyrosine-containing proteins characterised by specific affinity properties to substrates carrying amino groups, e.g. antigens or antibodies to polyallylamin surfaces [132, 133]. 375 376 377 378 379 380 381 382 Tyrosinase has shown activity on tyrosines of large polymers, such as wool fibres and silk fibroin that could be functionalized with different proteins, e.g. collagen, elastin and gelatine for acquiring bactericidal and fungicidal properties [134-136]. As a result of its activity on tyrosine-containing proteins and peptides, mushroom tyrosinase has also been applied to production of adhesives, starting from a polyphenolic protein with an enzyme to protein ratio of 5-50 units of enzyme per microgram of protein [85]. Moreover, the activity of mushroom tyrosinase (A. oryzae) in the presence of dopamine conferred adhesive properties to a diluted solution containing chitosan [137]. The viscosity of the solution increased with the progression of the reaction, probably due to the interaction of the quinone-like products of the enzymatic reaction with the 10 383 384 amino groups of chitosan. The adhesive strength of the enzyme-based preparation was higher than an analogous one prepared with the chemical cross-linker glutaraldehyde [137]. 385 386 387 388 389 390 391 392 393 394 395 396 397 398 399 400 401 402 403 404 405 406 5.6 Tyrosinase in food and feed applications Tyrosinase has been proposed for application in food processing not only for the melanogenic reaction it catalyses, e.g. in tea production [138], but also for its cross-linking activity that can modify the structure of food. Tyrosinases of different origins have been tested as cross-linking agents on a wide variety of proteins from milk, meat and cereals. In contrast to traditional cross-linking agents, tyrosinases are characterised by high specificity in the reaction they catalyse and, furthermore, they utilize food matrix components like proteins in their reaction and do not require the addition of any chemical or food additive. The improvement of the textural properties of food can be achieved by using carbohydrate-based gelling agents. Recently, a comparative study assessed the ability of different tyrosinases from fungi and bacteria, e.g. common mushroom A. bisporus, the plant pathogen Botryosphaeria obtusa, and the Gram-negative V. spinosum, to cross-link the commonly used gelling agent gelatine and revealed that the addition of phenolic compounds to the reaction mixture significantly accelerated the reaction [139]. Tyrosinase could be used for crosslinking the proteins of food matrices. For instance, in dairy and meat applications it can be applied in the production of low-calorie and low-fat food [140]. Caseins are generally good substrates for tyrosinase because their structure is flexible [141] and more accessible for the action of tyrosinase, e.g. the enzyme from V. spinosum [62]. The addition of tyrosinase from Trichoderma has been reported to improve the firmness of gels from raw milk and sodium caseinate [86]. This tyrosinase has also been reported to be able to form protein-oligosaccharide conjugates, where the protein was alpha-casein [142], but the reaction was not very efficient. Furthermore, this fungal tyrosinase has also been effective in improving the firmness of gels containing with low-meat chicken breast and created a network between the collagen molecules [143]. In baking, tyrosinase (Trichoderma) has also been able to enhance the hardness and reduce the extensibility of dough [144] and to modify the structure of baked bread by cross-linking the cereal proteins [145]. 407 408 409 The suggested application of tyrosinase to animal feed is quite recent. Aiming at the improvement of the nutritional value of animal feed, mushroom tyrosinase has recently been able to increase the bioavailability of iron from phytase treated fava bean-based preparations [146]. 410 411 412 413 414 415 416 417 418 419 420 421 6. Conclusion Many intracellular and secreted tyrosinases have been reported from bacteria, and four of them have already been subjects of patent applications. The production of novel bacterial tyrosinases has been eased by the increasing number of bacterial genomes sequenced. However, the identification of novel tyrosinases through genome mining studies is hindered by their strong sequence similarity with catechol oxidases. This review shows that a certain degree of sequence variation in residues and length is present even among reported and biochemically characterised tyrosinases. On the other hand, the classification of an enzyme according to its activity on typical tyrosinase substrates, i.e. an activity as mono- and diphenol oxidase could be misleading as some enzymes such as laccases have been reported to have tyrosinase activity. One of the advantages of bacterial tyrosinases is the ease of their production in recombinant form in a model host such as E. coli. This makes production in good quantities and protein engineering studies straight-forward and time-efficient and bears new potential for future applications. 11 422 423 424 425 Author’s contribution GF conducted the literature search and drafted the manuscript. KK, MS and LTM have contributed to the discussion and provided a critical evaluation of the information collected. All authors have read and approved the final article. 426 427 428 429 430 Acknowledgements GF was funded by TYROMAT, a project within the Empa Postdocs programme that is co-funded by the FP7: People Marie-Curie action COFUND. GF was also financially supported by the Finnish Cultural Foundation and Zerazyme, a project funded by the Finnish Agency for Technology and Innovation (funding decision 40161/10). We are grateful to Sarah Tighe-Jordan for language revision. 12 431 Figure legends 432 433 Figure 1 Oxidation of L-tyrosine to L-dopaquinone by tyrosinase. 434 435 436 437 Figure 2 Three-dimensional structure of tyrosinase from B. megaterium and characteristic sequence motifs of tyrosinases (PDB ID: 3MN8). The conserved sequence motifs identifying the copper A and B binding sites of tyrosinases are reported. Protein-specific variation in the distance between the conserved histidine residues is reported and X is any amino acid. 438 439 Figure 3 Biochemical properties of bacterial tyrosinases. 440 441 442 443 444 445 446 447 448 449 450 Molecular weight (MW, empty symbols), optimum pH (filled symbols) and temperature (grey symbols) of known bacterial tyrosinases belonging to the phyla of Actinobacteria, Firmicutes, Proteobacteria, Verrucomicrobia and Chloroflexi are shown. Whenever the optimum pH and temperature values were not available, the assay conditions for activity are reported (square symbol). Tyrosinases considered were from 1 Streptomyces antibioticus, 2 Streptomyces glaucescens, 3 Streptomyces nigrifaciens, 4 Streptomyces castaneoglobisporus, 5 Streptomyces lavendulae, 6 Streptomyces michiganensis, 7 Streptomyces sp. KY453, 8 Streptomyces albus, 9 Streptomyces sp. REN-21, 10 Bacillus megaterium, 11 Bacillus thuringiensis, 12 Marinomonas mediterranea, 13 Pseudomonas putida F6, 14-15 Ralstonia solanacearum, 16 Sinorhizobium meliloti, 17 Aeromonas media, 18 Pseudomonas sp. DSM13540, 19 Rhizobium etli CFN42, 20 Verrucomicrobium spinosum, 21 Thermomicrobium roseum. For details and references see Supplementary file 2. 451 452 453 Figure 4 Fields of application of tyrosinase based on the activity on phenolic compounds and the crosslinking activity. 454 Supplementary file 1 455 456 457 458 459 460 461 462 463 464 Sequence alignment of the characterised bacterial tyrosinases that are available at NCBI database (http://www.ncbi.nlm.nih.gov/). When significant, the consensus sequence (Jalview, http://www.jalview.org) is reported at the bottom of the alignment. Possible N-terminal arginine residues and the conserved cysteine residues in the copper A binding region are in grey. The C-terminal tyrosine motif and conserved sequence motifs involved in copper or oxygen binding are indicated and the key residues are indicated by a plus. Residues subject to mutagenesis and mentioned in the text are boxed. For details and references see Supplementary file 2. Tyrosinases considered were from 2 Streptomyces glaucescens, 4 Streptomyces castaneoglobisporus, 5 Streptomyces lavendulae, 10 Bacillus megaterium, 12 Marinomonas mediterranea, 14-15 Ralstonia solanacearum, 16 Sinorhizobium meliloti, 17 Aeromonas media, 19 Rhizobium etli CFN42, 20 Verrucomicrobium spinosum. 465 Supplementary file 2 466 467 Summary of the bacterial tyrosinases considered in this review, the reference to their sequence and to literature. 468 13 469 470 471 472 473 14 474 475 References 476 477 [1] Plonka PM, Grabacka M. Melanin synthesis in microorganisms — biotechnological and medical aspects. Acta Biochim Pol 2006;53:429–443. 478 479 480 [2] Geng J, Yu SB, Wan X, Wang XJ, Shen P, Zhou P, Chen XD. Protective action of bacterial melanin against DNA damage in full UV spectrums by a sensitive plasmid-based noncellular system. J Biochem Biophys Methods 2008;70:1151-1155. 481 482 [3] García-Rivera J, Casadevall A. Melanization of Cryptococcus neoformans reduces its susceptibility to the antimicrobial effects of silver nitrate. Medical mycology 2001;39:353-357. 483 484 485 [4] Geng J, Yuan P, Shao C, Yu SB, Zhou B, Zhou P, Chen XD. Bacterial melanin interacts with doublestranded DNA with high affinity and may inhibit cell metabolism in vivo. Arch Microbiol 2010;192:321329. 486 487 [5] Matoba Y, Kumagai T, Yamamoto A, Yoshitsu H, Sugiyama M. Crystallographic evidence that the dinuclear copper center of tyrosinase is flexible during catalysis. J Biol Chem 2006;281:8981-8990. 488 489 [6] Sendovski M, Kanteev M, Ben-Yosef VS, Adir N, Fishman A. First structures of an active bacterial tyrosinase reveal copper plasticity. J Mol Biol 2011;405:227-237. 490 491 492 [7] Ismaya WT, Rozeboom HJ, Weijn A, Mes JJ, Fusetti F, Wichers HJ, Dijkstra BW. Crystal structure of Agaricus bisporus mushroom tyrosinase: identity of the tetramer subunits and interaction with tropolone. Biochemistry 2011;50:5477-5486. 493 494 [8] Klabunde T, Eicken C, Sacchettini JC, Krebs B. Crystal structure of a plant catechol oxidase containing a dicopper center. Nat Struct Biol 1998;5:1084-1090. 495 496 497 [9] Virador VM, Reyes Grajeda JP, Blanco-Labra A, Mendiola-Olaya E, Smith GM, Moreno A, Whitaker JR. Cloning, sequencing, purification, and crystal structure of Grenache (Vitis vinifera) polyphenol oxidase. J Agric Food Chem 2010;58:1189-1201. 498 499 [10] Malviya N, Srivastava M, Diwakar SK, Mishra SK. Insights to sequence information of polyphenol oxidase enzyme from different source organisms. Appl Biochem Biotechnol 2011;165:397-405. 500 [11] Claus H, Decker H. Bacterial tyrosinases. Syst Appl Microbiol 2006;29:3-14. 501 502 [12] Fairhead M, Thöny-Meyer L. Bacterial tyrosinases: old enzymes with new relevance to biotechnology. New Biotechnology 2012;29:183-191. 503 504 [13] Popa C, Bahrim G. Streptomyces tyrosinase: production and practical applications. Innovative Romanian Food Biotechnology 2011; 8:1-7. 505 506 [14] Eisenman HC, Casadevall A. Synthesis and assembly of fungal melanin. Appl Microbiol Biotechnol 2012;93:931-940. 507 508 [15] Jang JH, Moon KD. Inhibition of polyphenol oxidase and peroxidase activities on fresh-cut apple by simultaneous treatment of ultrasound and ascorbic acid. Food Chem 2011;124:444-449. 15 509 510 511 [16] Jee JG, Park SJ, Kim HJ. Tyrosinase-induced cross-linking of tyrosine-containing peptides investigated by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Rapid Communications in Mass Spectrometry (RCM) 2000;14:1563-1567. 512 [17] Chang TS. An updated review of tyrosinase inhibitors. Int J Mol Sci. 2009;10:2440–2475. 513 514 [18] Solomon EI, Baldwin MJ, Lowery MD. Electronic structures of active sites in copper proteins: contributions to reactivity. Chem Rev 1992;92:521-542 515 516 [19] Betancourt AM, Bernan V, Herber W, Katz E. Analysis of tyrosinase synthesis in Streptomyces antibioticus. Microbiology 1992;138:787-794. 517 518 519 [20] Kohashi PY, Kumagai T, Matoba Y, Yamamoto A, Maruyama M, Sugiyama M. An efficient method for the overexpression and purification of active tyrosinase from Streptomyces castaneoglobisporus. Protein Expr Purif 2004;34:202-207. 520 521 [21] Fairhead M, Thöny-Meyer L. Role of the C-terminal extension in a bacterial tyrosinase. FEBS J 2010;277:2083-2095. 522 523 [22] Kawamura-Konishi Y, Maekawa S, Tsuji M, Goto H. C-terminal processing of tyrosinase is responsible for activation of Pholiota microspora proenzyme. Appl Microbiol Biotechnol 2010;90:227-234. 524 525 [23] Fujieda N, Murata M, Yabuta S, Ikeda T, Shimokawa C, Nakamura Y, Hata Y, Itoh S. Multifunctions of MelB, a fungal tyrosinase from Aspergillus oryzae. ChemBioChem 2012; 2011;13:193-201. 526 527 [24] Flurkey WH, Inlow JK. Proteolytic processing of polyphenol oxidase from plants and fungi. J Inorg Biochem. 2008;102:2160-70. 528 529 [25] Nambudiri AM, Bhat JV, Rao PV. Enzymic conversion of p-coumarate into caffeate by Streptomyces nigrifaciens. Biochem.J. 1972;128:63. 530 531 [26] Liu N, Zhang T, Wang YJ, Huang YP, Ou JH, Shen P. A heat inducible tyrosinase with distinct properties from Bacillus thuringiensis. Lett Appl Microbiol 2004;39:407-412. 532 533 [27] Fernandez E, Sanchez-Amat A, Solano F. Location and catalytic characteristics of a multipotent bacterial polyphenol oxidase. Pigment Cell Research 1999;12:331-339. 534 535 [28] Beltramini M, Lerch K. Fluorescence properties of Neurospora tyrosinase. Biochem J 1982;205:173180. 536 537 538 [29] Gasparetti C, Faccio G, Arvas M, Buchert J, Saloheimo M, Kruus K. Discovery of a new tyrosinaselike enzyme family lacking a C-terminally processed domain: production and characterization of an Aspergillus oryzae catechol oxidase. Appl Microbiol Biotechnol 2010;86:213-226. 539 540 [30] Jackman MP, Hajnal A, Lerch K. Albinomutants of Streptomyces glaucescens tyrosinase. Biochem J 1991;274:707-713. 541 542 [31] Huber M, Lerch K. Identification of two histidines as copper ligands in Streptomyces glaucescens tyrosinase. Biochemistry 1988;27:5610-5615. 543 544 [32] Marusek CM, Trobaugh NM, Flurkey WH, Inlow JK. Comparative analysis of polyphenol oxidase from plant and fungal species. J Inorg Biochem 2006;100:108-123. 16 545 546 [33] Bustos Arcos VP. Produccion de melaninas en microorganismos recombinantes 2005; Mex. Patent. MXPA04004786A. 04.05.20. 547 548 [34] Stephanopoulos G, Santos CNS. Methods for identifying bacterial strains that produce L-tyrosine. United States Patent Appl US0151496 A1. 09.02.26. 549 550 [35] Santos CNS, Stephanopoulos G. Melanin-based high-throughput screen for L-tyrosine production in Escherichia coli. Appl Environ Microbiol. 2008 ;74:1190–1197. 551 552 [36] Shuster BYV, Sendovski M, Fishman A. Directed evolution of tyrosinase for enhanced monophenolase/diphenolase activity ratio. Enzyme Microb Technol 2010;47:372-376. 553 554 [37] Jackman MP, Huber M, Hajnal A, Lerch K. Stabilization of the oxy form of tyrosinase by a single conservative amino acid substitution. Biochem. J. 1992;282:915-918. 555 556 [38] Miller KI, Cuff ME, Lang WF, Varga-Weisz P, Field KG, van Holde KE. Sequence of the Octopus dofleini hemocyanin subunit: structural and evolutionary implications. J Mol Biol 1998;278:827-842. 557 558 559 [39] Rodríguez-López JN, Fenoll LG, García-Ruiz PA, Varón R, Tudela J, Thorneley RN, García-Cánovas F. Stopped-flow and steady-state study of the diphenolase activity of mushroom tyrosinase. Biochemistry 2000;39:10497-506. 560 561 [40] Hirota S, Kawahara T, Lonardi E, de Waal E, Funasaki N, Canters GW. Oxygen binding to tyrosinase from Streptomyces antibioticus studied by laser flash photolysis. J Am Chem Soc 2005;127:17966-7. 562 563 [41] Liaw LL, Lee YH. Histidine residues 102 and 117 of MelC1 play different roles in the chaperone function for Streptomyces apotyrosinase. Biochem Biophys Res Commun 1995;214:447-453. 564 565 [42] Lopez-Serrano D, Solano F, Sanchez-Amat A. Identification of an operon involved in tyrosinase activity and melanin synthesis in Marinomonas mediterranea. Gene 2004;342:179-187. 566 567 [43] Hernández-Romero D, Sanchez-Amat A, Solano F. A tyrosinase with an abnormally high tyrosine hydroxylase/dopa oxidase ratio. FEBS J 2012; 273:257-270. 568 569 [44] Kong KH, Hong MP, Choi SS, Kim YT, Cho SH. Purification of Thermomicrobium roseum tyrosinase. Biotechnol Appl Biochem 2000;31:113-118. 570 571 [45] Hintermann G, Zatchej M, Hütter R. Cloning and expression of the genetically unstable tyrosinase structural gene from Streptomyces glaucescens. Mol Gen Genet 1985;200:422-432. 572 573 574 [46] Ikeda K, Masujima T, Suzuki K, Sugiyama M. Cloning and sequence analysis of the highly expressed melanin-synthesizing gene operon from Streptomyces castaneoglobisporus. Appl Microbiol Biotechnol 1996;45:80-85. 575 576 [47] Dolashki A, Gushterova A, Voelter W, Tchorbanov B. Purification and characterization of tyrosinases from Streptomyces albus. Z Naturforsch C 2009;64:724-732. 577 578 [48] Shuster V, Fishman A. Isolation, cloning and characterization of a tyrosinase with improved activity in organic solvents from Bacillus megaterium. J Mol Microbiol Biotechnol 2009;17:188-200. 579 580 581 [49] Mercado-Blanco J, Garcia F, Fernandez-Lopez M, Olivares J. Melanin production by Rhizobium meliloti GR4 is linked to nonsymbiotic plasmid pRmeGR4b: cloning, sequencing, and expression of the tyrosinase gene mepA. J Bacteriol 1993;175:5403-5410. 17 582 583 584 [50] Wan X, Chai B, Liao Y, Su Y, Ye T, Shen P, Chen X. Molecular and biochemical characterization of a distinct tyrosinase involved in melanin production from Aeromonas media. Appl Microbiol Biotechnol 2009;82:261-269. 585 586 587 [51] Cabrera-Valladares N, Martinez A, Pinero S, Lagunasmunoz V, Tinoco R, Deanda R, Vazquezduhalt R, Bolivar F, Gosset G. Expression of the melA gene from Rhizobium etli CFN42 in Escherichia coli and characterization of the encoded tyrosinase. Enzyme Microb Technol 2006;38:772-779. 588 589 [52] Bendtsen JD, Nielsen H, Widdick D, Palmer T, Brunak S. Prediction of twin-arginine signal peptides. BMC Bioinformatics 2005; 6:167. 590 591 [53] Lerch K. Primary structure of tyrosinase from Neurospora crassa. II. Complete amino acid sequence and chemical structure of a tripeptide containing an unusual thioether. J Biol Chem 1982;257:6414-6419. 592 593 594 595 [54] Gielens C, DE Geest N, Xin X, Devreese B, Van Beeumen J, Preaux G. Evidence for a cysteinehistidine thioether bridge in functional units of molluscan haemocyanins and location of the disulfide bridges in functional units d and g of the c-haemocyanin of Helix pomatia. Eur J Biochem 1997;248:879888. 596 597 [55] Monnet V. Bacterial oligopeptide-binding proteins. Cellular and molecular life sciences: CMLS 2003;60:2100-2114. 598 599 [56] Surwase SN, Patil SA, Apine OA, Jadhav JP. Efficient microbial conversion of L-tyrosine to L-DOPA by Brevundimonas sp. SGJ. Appl.Biochem.Biotechnol. 2012;In press. 600 601 [57] Yang HY, Chen CW. Extracellular and intracellular polyphenol oxidases cause opposite effects on sensitivity of Streptomyces to phenolics: a case of double-edged sword. PLoS One 2009;4:e7462. 602 603 [58] Lopez-Serrano D, Solano F, Sanchez-Amat A. Involvement of a novel copper chaperone in tyrosinase activity and melanin synthesis in Marinomonas mediterranea. Microbiology 2007;153:2241-2249. 604 605 [59] Ito M, Oda K. An organic solvent resistant tyrosinase from Streptomyces sp. REN-21: purification and characterization. Biosci Biotech Bioch 2000;64:261-267. 606 607 [60] Goldfeder M, Egozy M, Shuster BYV, Adir N, Fishman A. Changes in tyrosinase specificity by ionic liquids and sodium dodecyl sulphate. Appl Microbiol Biotechnol 2012:In press. 608 609 610 [61] Selinheimo E, NiEidhin D, Steffensen C, Nielsen J, Lomascolo A, Halaouli S, Record E, O'Beirne D, Buchert J, Kruus K. Comparison of the characteristics of fungal and plant tyrosinases. J Biotechnol 2007;130:471-480. 611 612 [62] Fairhead M, Thöny-Meyer L. Cross-linking and immobilisation of different proteins with recombinant Verrucomicrobium spinosum tyrosinase. J Biotechnol 2010;150:546-551. 613 614 [63] Ikeda K, Masujima T, Sugiyama M. Effects of methionine and Cu2+ on the expression of tyrosinase activity in Streptomyces castaneoglobisporus. J Biochem 1996;120:1141-1145. 615 616 [64] Mayer AM. Polyphenol oxidases in plants and fungi: going places? A review. Phytochemistry 2006;67:2318-2331. 18 617 618 619 [65] Soler-Rivas C, Möller AC, Arpin N, Olivier JM, Wichers HJ. Induction of a tyrosinase mRNA in Agaricus bisporus upon treatment with a tolaasin preparation from Pseudomonas tolaasii. Physiol Mol Plant Pathol 2001;58:95-99. 620 621 [66] Kanda S, Aimi T, Masumoto S, Nakano K, Kitamoto Y, Morinaga T. Photoregulated tyrosinase gene in Polyporus arcularius. Mycoscience 2007;48:34-41. 622 623 [67] Gukasyan GS. Effect of lignin on growth and tyrosinase activity of fungi from the genus Aspergillus. Biochemistry (Moscow) 1999;2012:223-227. 624 625 [68] Gruhn CM, Miller Jr OK. Effect of copper on tyrosinase activity and polyamine content of some ectomycorrhizal fungi. Mycol Res 1991;95:268-272. 626 627 [69] Philipp S, Held T, Kutzner HJ. Purification and characterization of the tyrosinase of Streptomyces michiganensis DSM 40015. J Basic Microbiol 1991;31:293-300. 628 629 [70] Held T, Kutzner HJ. Genetic recombination in Streptomyces michiganensis DSM 40,015 revealed three genes responsible for the formation of melanin. J Basic Microbiol 1991;31:127-134. 630 631 [71] Horowitz NH, Fling M, Feldman HM, Pall ML, Froehner SC. Derepression of tyrosinase synthesis in Neurospora by amino acid analogs. Dev Biol 1970;21:147-156. 632 633 634 [72] Wichers HJ, Recourt K, Hendriks M, Ebbelaar CE, Biancone G, Hoeberichts FA, Mooibroek H, SolerRivas C. Cloning, expression and characterisation of two tyrosinase cDNAs from Agaricus bisporus. Appl Microbiol Biotechnol 2003;61:336-341. 635 636 637 [73] Bubacco1 L, Vijgenboom E, Gobin C, Tepper AWJW, Salgado J, Canters GW. Kinetic and paramagnetic NMR investigations of the inhibition of Streptomyces antibioticus tyrosinase. J Mol Catal BEnzym 2000;8: 27–35. 638 639 [74] Ito M, Inouye K. Catalytic properties of an organic solvent-resistant tyrosinase from Streptomyces sp. REN-21 and its high-level production in E. coli. J Biochem 2005;138:355-362. 640 641 [75] Wang G, Aazaz A, Peng Z, Shen P. Cloning and overexpression of a tyrosinase gene mel from Pseudomonas maltophila. FEMS Microbiol Lett 2000;185:23-27. 642 643 [76] Lagunas-Muῆoz VH, Cabrera-Valladares N, Bolıvar F, Gosset G, Martınez A. Optimum melanin production using recombinant Escherichia coli. J Appl Microb 2006;101:1002-1008. 644 645 [77] Nishimura I. Acceleration of oxidation reaction of monophenols using tyrosinase. Jpn. Patent. JP 53121995. 77.03.29. 646 [78] Fujiwara H. Method for crosslinking polypeptide. Jpn. Patent. JP 2005-287302. 02.04.03. 647 [79] Fujiwara H. Process for crosslinking polypeptides. Patent. Pub. No. WO/2003/082923. 02.08.09. 648 649 650 [80] Jadhav JP, Phugare SS, Dhanve RS, Jadhav SB. Rapid biodegradation and decolorization of Direct Orange 39 (Orange TGLL) by an isolated bacterium Pseudomonas aeruginosa strain BCH. Biodegradation 2010;21:453-463. 651 652 [81] Lantto R, Niku-Paavola M, Schonberg C, Buchert J. A tyrosinase enzyme. Intern. Publ. Num. WO 02/14484 A1. 02.02.21. 19 653 654 [82] Thöny-Meyer LC, Fairhead M. Polypeptides having tyrosinase activity and uses thereof. Int. Patent Appl. WO 2011/000121 A1. 10.07.01. 655 656 657 [83] della-Cioppa GR, Garger, SJ, Holtz RB, McCulloch MJ, Sverlow GG. Method for making stable, extracellular tyrosinase and synthesis of polyphenolc polymers therefrom. United States Patent. US 5486351. 96.01.23. 658 659 660 [84] Paget MS, Hintermann G, Smith CP. Construction and application of streptomycete promoter probe vectors which employ the Streptomyces glaucescens tyrosinase-encoding gene as reporter. Gene 1994;146:105-110. 661 662 [85] Benedict CV. Picciano PT. Method for making dopa-containing bioadhesive proteins from tyrosinecontaining proteins. Eur. Patent. EP 0242656 A2. 87.02.04. 663 664 [86] Ercili Cura D, Lille M, Partanen R, Kruus K, Buchert J, Lantto R. Effect of Trichoderma reesei tyrosinase on rheology and microstructure of acidified milk gels. Int Dairy J 2010;20:830-837. 665 666 [87] Carvalho GMJ, Alves TLM, Freire DMG. L-DOPA production by immobilized tyrosinase. Appl Biochem Biotechnol 2000;84-86:791-800. 667 668 [88] Xu D, Chen J, Yang Z. Use of cross-linked tyrosinase aggregates as catalyst for synthesis of L-DOPA. Biochem Eng J 2012;63:88-94. 669 670 [89] Durána N, Rosa MA, D’Annibale A, Gianfreda L Applications of laccases and tyrosinases (phenoloxidases) immobilized on different supports: a review. Enzyme Microb Tech 2002;31:907–931 671 672 [90] Seetharam GB, Saville BA. Degradation of phenol using tyrosinase immobilized on siliceous supports. Water Res. 2003;2012:436-440. 673 674 [91] Tuncagil S, Kayahan SK, Bayramoglu G, Arica MY, Toppare L. L-DOPA synthesis using tyrosinase immobilized on magnetic beads. J Molec Catal B 2009;58:187-193. 675 676 [92] Tembe S, Karve M, Inamdar S, Haram S, Melo J, D’Souza SF. Development of electrochemical biosensor based on tyrosinase immobilized in composite biopolymeric film. Anal Biochem 2006;349:72-77. 677 678 [93] Naidja A, Huang PM, Bollag JM. Activity of tyrosinase immobilized on hydroxyaluminummontmorillonite complexes. Journal of Molecular Catalysis A: Chemical 1997;115:305-316. 679 680 [94] Pialis P, Saville BA. Production of l-DOPA from tyrosinase immobilized on nylon 6,6: enzyme stability and scaleup. Enzyme Microb Technol 1998;22:261-268. 681 682 [95] Seetharam G, Saville BA. L-DOPA production from tyrosinase immobilized on zeolite. Enzyme Microb Technol 2002;31:747-753. 683 [96] Peek SM. Method of dyeing animal fibres. United States Patent. US 2539202. 47.12.11. 684 685 [97] Warner JC, Stoler EJ. Color composition containing an aromatic compound and tyrosinase. United States Patent. Appl. US 2011/0113571 A1. 10.11.15. 686 687 [98] Yoshio T, Yoshiharu Y, Kuniaki S. Hair coloring and waiving using oxidases. J Society of Cosmetic Chemists 1991;2012:282. 20 688 689 [99] Abdullah J, Ahmad M, Karuppiah N, Heng LY, Sidek H. Immobilization of tyrosinase in chitosan film for an optical detection of phenol. Sensors Actuators B: Chem 2006;114:604-609. 690 691 [100] Wang G, Xu J, Ye L, Zhu J, Chen H. Highly sensitive sensors based on the immobilization of tyrosinase in chitosan. Bioelectrochemistry 2002;57:33-38. 692 693 694 [101] Streffer K, Vijgenboom E, Tepper AWJW, Makower A, Scheller FW, Canters GW, Wollenberger U. Determination of phenolic compounds using recombinant tyrosinase from Streptomyces antibioticus. Anal Chim Acta 2001;427:201-210. 695 696 [102] Tatsuma T, Komori K, Yeoh H, Oyama N. Disposable test plates with tyrosinase and β-glucosidases for cyanide and cyanogenic glycosides. Anal Chim Acta 2000;408:233-240. 697 698 [103] Girelli AM, Giuliani T, Mattei E, Papaleo D. Determination of an antioxidant capacity index by immobilized tyrosinase bioreactor. J Agric Food Chem 2009;57:5178-5186. 699 700 [104] Abhijith KS, Kumar PV, Kumar MA, Thakur MS. Immobilised tyrosinase-based biosensor for the detection of tea polyphenols. Anal Bioanal Chem 2007;389:2227-2234. 701 702 [105] Karim F, Fakhruddin ANM. Recent advances in the development of biosensor for phenol: a review. Rev Environ Sci Biotech, 2012: In press. 703 704 705 [106] Nolan LC, O’Connor KE. Use of Pseudomonas mendocina, or recombinant Escherichia coli cells expressing toluene-4-monooxygenase, and a cell-free tyrosinase for the synthesis of 4-fluorocatechol from fluorobenzene. Biotechnol Lett 2007;7:1045-1050. 706 [107] Huynh T. The Parkinson's disease market. Nature Reviews Drug Discovery 2011;10:571-572. 707 708 [108] Wykes JR, Dunnill P, Lilly MD. Conversion of tyrosine to L-dihydroxyphenylalanine using immobilized tyrosinase. Nature New Biology 1971;230:187-187. 709 710 [109] de Faria RO, Moure VR, Lopes de Almeida Amazonas MA, Krieger N, Mitchell DA. The biotechnological potential of mushroom tyrosinases. Food Technol Biotechnol 2007;45:287–294. 711 712 [110] Park HS, Lee JY, Kim HS. Production of L-DOPA(3,4-dihydroxyphenyl-L-alanine) from benzene by using a hybrid pathway. Biotechnol Bioeng 1998;58:339-43. 713 714 [111] Ito S, Prota G. A facile one-step synthesis of cysteinyldopas using mushroom tyrosinase. Experientia 1977;33:1118-1119. 715 716 [112] Pandey G, Muralikrishna C, Bhalerao UT. Mushroom tyrosinase catalysed synthesis of coumestans, bebzofuran derivatives and related heterocyclic compounds. Tetrahedron 1989;45:6867-6874. 717 718 719 [113] Espin de Gea JC, De Tomas Barberan FA, Garcia Viguera MC, Ferreres De Arce F, Soler Rivas C, Wichers H. Enzymatic synthesis of antioxidant hydroxytyrosol. United States Patent. Appl. 20030180833 A1.03.02.11. 720 721 [114] Espín JC, Soler-Rivas C, Cantos E, Tomás-Barberán FA, Wichers JH. Synthesis of the antioxidant hydroxytyrosol using tyrosinase as biocatalyst. J Agric Food Chem 2001:49:1187–1193 722 723 [115] Baranowitz SA. Methods for treating wounds using melanin and related substances. Intern. Publ. Num. WO2003051331A1. 02.10.28. 21 724 725 [116] Berliner DL, Erwin RL, Mcgee DR. Therapeutic uses of melanin. United States Patent. US 5703051. 98.07.07. 726 727 728 [117] Greggio E, Bergantino E, Carter D, Ahmad R, Costin GE, Hearing VJ, Clarimon J, Singleton A, Eerola J, Hellström O, Tienari PJ, Miller DW, Beilina A, Bubacco L, Cookson MR. Tyrosinase exacerbates dopamine toxicity but is not genetically associated with Parkinson's disease. J Neurochem 2005;93:246-256. 729 730 731 [118] Lopukhin JM, Zuevsky VV, Rabovsky AB, Andrianov IP. Method for visual indication of cholesterol on skin surface agents used therefor and methods for producing such agents. United States Patent. US 5489510 A. 93.12.09. 732 733 734 [119] Marino SM, Fogal S, Bisaglia M, Moro S, Scartabelli G, De Gioia L, Spada A, Monzani E, Casella L, Mammi S, Bubacco L. Investigation of Streptomyces antibioticus tyrosinase reactivity toward chlorophenols. Arch Biochem Biophys 2011;505:67-74. 735 736 [120] Battaini G, Monzani E, Casella L, Lonardi E, Tepper AW, Canters GW, Bubacco L. Tyrosinasecatalyzed oxidation of fluorophenols. J Biol Chem 2002;277:44606-44612. 737 738 [121] Saratale RG, Saratale GD, Chang JS, Govindwar SP. Bacterial decolorization and degradation of azo dyes: A review. J Taiwan Inst Chem E 2011;42:138-157. 739 740 [122] Duran N, Esposito E. Potential applications of oxidative enzymes and phenoloxidases-like compoundsin wastewater and soil treatment: a review. Appl Catalysis B: Environ 2000;28:83-99. 741 742 743 [123] Sani S, Muhid MNM, Hamdan H. Design, synthesis and activity study of tyrosinase encapsulated silica aerogel (TESA) biosensor for phenol removal in aqueous solution. J Sol Gel Sci Technol 2011;59:718. 744 745 [124] Wada S, Ichikawa H, Tatsumi K. Removal of phenols from wastewater by soluble and immobilized tyrosinase. Biotechnol Bioeng 1993;42:854-858. 746 747 748 [125] Yamada K, Akiba Y, Shibuya T, Kashiwada A, Matsuda K, Hirata M. Water purification through bioconversion of phenol compounds by tyrosinase and chemical adsorption by chitosan beads. Biotechnol Prog 2008;21:823-829. 749 750 751 [126] Pajot HF, Fariña JI, de Figueroa LIC. Evidence on manganese peroxidase and tyrosinase expression during decolorization of textile industry dyes by Trichosporon akiyoshidainum. Int Biodeterior Biodegrad 2011;65:1199-1207. 752 753 [127] Saratale RG, Saratale GD, Chang JS, Govindwar SP. Decolorization and biodegradation of textile dye Navy blue HER by Trichosporon beigelii NCIM-3326. J Hazard Mater 2009;166:1421-1428. 754 755 756 [128] Chen T, Vazquez-Duhalt R, Wu CF, Bentley WE, Payne GF. Combinatorial screening for enzymemediated coupling. Tyrosinase-catalyzed coupling to create protein-chitosan conjugates. Biomacromolecules 2001;2:456-462. 757 758 759 [129] Demolliens A, Boucher C, Durocher Y, Jolicoeur M, Buschmann MD, De Crescenzo G. Tyrosinasecatalyzed synthesis of a universal coil-chitosan bioconjugate for protein immobilization. Bioconjug Chem 2008;19:1849-1854. 760 761 [130] Anghileri A, Lantto R, Kruus K, Arosio C, Freddi G. Tyrosinase-catalyzed grafting of sericin peptides onto chitosan and production of protein-polysaccharide bioconjugates. J Biotechnol 2007;127:508-519. 22 762 763 [131] Kang GD, Lee KH, Ki CS, Nahm JH, Park YH. Silk fibroin/chitosan conjugate crosslinked by tyrosinase. Macromol Research 2004;12:534-539. 764 765 [132] Barbari TA, Ahmed SR. Affinity membrane for capture of a target biomolecule and formation thereof by site-directed immobilization of a capture molecule. United States Patent. US 2006/0292680 A1. 10.08.10. 766 767 [133] Ahmed SR, Lutes AT, Barbari TA. Specific capture of target proteins by oriented antibodies bound to tyrosinase-immobilized Protein A on a polyallylamine affinity membrane surface. J Mem Sci 2006;311-321. 768 769 [134] Jus S, Kokol V, Guebitz GM. Tyrosinase-catalysed coating of wool fibres with different protein-based biomaterials. J Biomater Sci Polym Ed. 2009;20(2):253-69. 770 771 772 [135] Freddi G, Anghileri A, Sampaio S, Buchert J, Monti P, Taddei P. Tyrosinase-catalyzed modification of Bombyx mori silk fibroin: Grafting of chitosan under heterogeneous reaction conditions. J Biotech 2006;125:281–294 773 774 [136] Lantto R, Heine E, Freddi G, Lappalainen A, Miettinen-Oinonen A, Niku-Paavola ML, Buchert J. Enzymatic modification of wool with tyrosinase and peroxidase. J Textile Institute2005; 96:109-116. 775 776 [137] Yamada K, Aoki T, Ikeda N, Hirata M, Hata Y, Higashida K, Nakamura Y. Application of chitosan solutions gelled by melB tyrosinase to water-resistant adhesives. J Appl Polym Sci 2008;107:2723-2731. 777 778 [138] Slaga TJ, Zhao J. Processing method for manufacturing black tea and an improved black tea. United States Patent US 6602527 B1. 00.11.22. 779 780 [139] Jus S, Stachel I, Fairhead M, Meyer M, Thöny-Meyer L, Guebitz GM. Enzymatic cross-linking of gelatine with laccase and tyrosinase. Biocatal Biotransfor 2012;30:86-95. 781 782 [140] Onwulata CI, Tomasula PM. Gelling properties of tyrosinase-treated dairy proteins. Food Bioprocess Tech 2008; 2010;3:554-560. 783 784 [141] David SH. Casein structure, self-assembly and gelation. Curr Opin Colloid Interface Sci 2002;7:456461. 785 786 [142] Selinheimo E, Lampila P, Mattinen ML, Buchert J. Formation of protein-oligosaccharide conjugates by laccase and tyrosinase. J Agric Food Chem 2008;56:3118-3128. 787 788 789 [143] Lantto R, Puolanne E, Kruus K, Buchert J, Auutio K. Tyrosinase-aided protein cross-linking: effects on gel formation of chicken breast myofibrils and texture and water-holding of chicken breast meat homogenate gels. J Agric Food Chem 2007;55:1248-1255. 790 791 [144] Selinheimo E, Autio K, Kruus K, Buchert J. Elucidating the mechanism of laccase and tyrosinase in wheat bread making. J Agric Food Chem 2007;55:6357-6365. 792 793 [145] Takasaki S, Kawakishi S, Murata M, Homma S. Polymerisation of gliadin mediated by mushroom tyrosinase. Lebensm Wiss Technol 2001;34:507-512. 794 795 [146] Luo YW, Xie WH, Xu M, Luo FX. Effects of phytase and polyphenol oxidase treatments on in vitro iron bioavailability in faba bean (Vicia faba L.). CyTA - Journal of Food 2012:1-7. 23 796 797 798 [147] Matoba Y, Bando N, Oda K, Noda M, Higashikawa F, Kumagai T, Sugiyama M. A molecular mechanism for copper transportation to tyrosinase that is assisted by a metallochaperone, caddie protein. J Biol Chem 2011;286:30219-30231. 799 800 [148] Katz E, Betancourt A. Induction of tyrosinase by L-methionine in Streptomyces antibioticus. Can J Microbiol 1988;34:1297-1303 801 802 [149] Lerch K, Ettinger L. Purification and characterization of a tyrosinase from Streptomyces glaucescens. Eur J Biochem 1972;31:427-437. 803 804 [150] Held T, Kutzner HJ. Transcription of the tyrosinase gene in Streptomyces michiganensis DSM 40015 is induced by copper and repressed by ammonium. Microbiology 1990;136:2413-2419. 805 806 [151] Coyne VE, al-Harthi L. Induction of melanin biosynthesis in Vibrio cholerae. Appl Environ Microbiol 1992;58:2861-2865. 807 808 [152] Uchisawa H, Naraoka T, Takatani Y, Abe K, Yamaguchi SI, Ichida J, Matsue H. Production of watersoluble melanin dyestuff. Jpn. Patent. JP 1996-038187. 94.08.03. 809 [153] Funayama M, Shiyouen S, Yamamoto R. Dyeing of fiber. Jpn. Patent. JP 1997-087977. 97.03.31. 810 [154] Herlihy WC. Skin tanning composition and method. United States Patent. US 4515773. 07.05.1985. 811 812 [155] Moon SH, Kim GY. Tyrosinase enzyme electrode and production method thereof. United States Patent US 7935233. 11.05.03. 813 814 [156] Arter TC. Multilayer analytical element for salicylate assay. United States Patent. US 5474907. 94.03.25. 815 816 [157] Daniel DS, Method and element for assay of catechol and catechol generating substances. Eur. Patent. EP 0464934 A1. 91.06.28. 817 818 [158] Bouvrette P, Luong JHT. A coupled enzymatic assay for salicylate and acetylsalicylate using salicylate hydroxylase and tyrosinase. Anal Chim Acta, 1996;335:1 69-175 819 820 821 [159] Nolan CL, O’Connor KE. A spectrophotometric method for the quantification of an enzyme activity producing 4-substituted phenols: Determination of toluene-4-monooxygenase activity. Anal Biochem 2005;344:224–231. 822 823 [160] Campanella L, Gregori E, Tomassetti M. Salicylic acid determination in cow urine and drugs using a bienzymatic sensor. J Pharm Bioml Anal 2006;42:94–99 824 825 826 [161] Schweitzer AD, Revskaya E, Chu P, Pazo V, Friedman M, Nosanchuk JD, Cahill S, Frases S, Casadevall A, Dadachova E. Melanin-covered nanoparticles for protection of bone marrow during radiation therapy of cancer. Int J Radiat Oncol 2010;78:1494–1502. 827 [162] Dantas-Miranda AL. Mosquito deterrent. Patent. WO 2006021834 A1. 04.08.27. 828 829 [163] Tatsumi K, Takaoichikawa HS. Treatment of chlorophenol-containing water. Jpn. Patent. JP 09239396. 96.03.07. 830 831 [164] Chiacchierini E. Bioremediation of food industry effluents: recent applications of free and immobilised polyphenoloxidases. Food Sci Technol Int 2004;10:373-382. 24 832 833 [165] Atlow SC, Bonadonna-Aparo L, Klibanov AM. Dephenolization of industrial wastewaters catalyzed by polyphenol oxidase. Biotechnol Bioeng 1984;26:599-603. 834 [166] Nakamoto S. Method for removing phenols. Jpn. Patent. JP 05-115883. 91.10.25. 835 836 [167] Bevilaqua JV, Cammarota MC, Freire DMG, Sant'Anna. GL. Phenol removal through combined biological and enzymatic treatments. Brazil J Chem Eng 2002;19:151-158. 837 [168] Asai J. Method for removing COD in drainage water. Jpn. Patent. JP 09-029264. 95.07.17 838 839 [169] Kalme SD, Parshetti GK, Jadhav SU, Govindwar SP. Biodegradation of benzidine based dye Direct Blue-6 by Pseudomonas desmolyticum NCIM 2112. Bioresour Technol 2007;98:1405-1410. 840 841 [170] Kalme S, Ghodake G, Govindwar S. Red HE7B degradation using desulfonation by Pseudomonas desmolyticum NCIM 2112. Int Biodeterior Biodegrad 2007;60:327-333. 842 843 844 [171] Oturkar CC, Nemade HN, Mulik PM, Patole MS, Hawaldar RR, Gawai KR. Mechanistic investigation of decolorization and degradation of Reactive Red 120 by Bacillus lentus BI377. Bioresour Technol 2011;102:758-764. 845 846 [172] Gandía-Herrero F, Escribano J, García-Carmona F. Characterization of the activity of tyrosinase on betanidin. J Agric Food Chem 2007;55:1546-1551. 847 848 849 [173] Della-Cioppa GR, Garger SJ, Holtz RB, McCulloch MJ, Sverlow GG. Method for making stable, extracellular tyrosinase and synthesis of polyphenolic polymers therefrom. United States Patent. US 5466592A. 93.12.14. 850 [174] Miyakoshi T. Coating composition. Jpn. Patent. JP 05-117591 A. 91.12.29. 851 [172] Nishimatsu M. Film forming method. Jpn Patent. JP 63-294967 A. 87.05.26. 852 [175] Ellis J, Cornell DL. A method for treating proteinaceous fibres. Eur. Patent EP 1311 719 B1. 01.08.15 853 854 [176] Ellis J, Cornell DL. Treating proteinaceous materials - precision processes textiles limited. Int. Patent. WO/2003/069051. 03.02.10. 855 856 [177] Zhao J, Slaga TJ, Fields C. High yield process for producing theaflavins and products of such process. United States Patent. US 20040137129 A1. 03.11.18. 857 858 [178] Tu YY, Xu XQ, Xia HL, Watanabe N. Optimization of theaflavin biosynthesis from tea polyphenols using an immobilized enzyme system and response surface methodology. Biotech Lett 2005;27:269–274 859 860 [179] Autio K, Lille M, Myllarinen P. Milk product and method for its preparation. WIPO Patent Appl. WO/2007/141385 A1. 07.06.05. 861 [180] Uchiyama S. Method and reagent for counting microorganism. Jpn. Patent. JP 06-113892 A. 92.10.01. 862 863 [181] Bernan V, Filpula D, Herber W, Bibb M, Katz E. The nucleotide sequence of the tyrosinase gene from Streptomyces antibioticus and characterization of the gene product. Gene 1985;37:101-110 864 865 [182] Kawamoto S, Nakamura M, Yashima S. Cloning, sequence and expression of the tyrosinase gene from Streptomyces lavendulae MA406 A-1. J Ferment Bioeng 1993;76:345-355. 25 866 867 [183] Yoshimoto T, Yamamoto K, Tsuru D. Extracellular tyrosinase from Streptomyces sp. KY-453: purification and some enzymatic properties. J Biochem 1985;97:1747-1754. 868 869 870 [184] Sanchez-Amat A, Lucas-Elı́o P, Fernández E, Garcı́a-Borrón JC, Solano F. Molecular cloning and functional characterization of a unique multipotent polyphenol oxidase from Marinomonas mediterranea. BBA-Protein Struct M 2001;1547:104-116. 871 872 873 [185] McMahon AM, Doyle EM, Brooks S, O’Connor KE. Biochemical characterisation of the coexisting tyrosinase and laccase in the soil bacterium Pseudomonas putida F6. Enzyme Microb Tech 2007;40:14351441. 874 875 876 [186] Pinero S, Rivera J, Romero D, Cevallos MA, Martinez A, Bolivar F, Gosset G. Tyrosinase from Rhizobium etli is involved in nodulation efficiency and symbiosis-associated stress resistance. J Mol Microbiol Biotechnol 2007;13:35-44. 877 26 ● Figure 1 878 Figure 1 879 27 T optimum Temperature optimum ( C) Molecular weight (kDa) 120 Actinobacteria pH optimum 12 Chloroflexi Verrucomicrobia 10 Firmicutes Proteobacteria 100 8 80 6 60 4 pH optimum MW 140 40 2 20 0 0 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 Identifier Figure 3 880 881 882 883 Supplementary file 1 884 885 886 887 888 889 890 891 892 2 4 5 10 16 20 15 ------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------ 28 893 894 895 896 897 898 899 900 901 902 903 904 905 906 907 908 909 910 911 912 913 914 915 916 917 918 919 920 921 922 923 924 925 926 927 928 929 19 14 12 17 MPWLVGKPSLERSWNAILSFPESGFQLECRNTIGSSVFSSHFTLHFRVARRLLHFSCRRF ------------------------------MRIDFTINNGGDAAARYLTWAPSPLRLRLL ----------------------------------------------------------------------------------------------------------------------- 1 2 4 5 10 16 20 15 19 14 12 17 -------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------MSPPTTSRRQFLVTAGA ----------------------------------------------MVVRRTVLKAIAGT TETQKEPTQALWWCELPTAPAPRRRGTGLKAALILAKDNSNPRESKMSITRRHVIVQGGV DATPGPDVVATLSEDRQPNGGSIRFCATPDGNFTPTLKVPLPASGASVTVYVRGKFGTPS ------------------------------------------------MIRVRKNVNELT -------------------------------------------------------MKKGL 1 2 4 5 10 16 20 15 19 14 12 17 Cons -------------------MT----VRKNQASLTAEEKRRFVAALLELKR--TGRYDAFV -------------------MT----VRKNQATLTADEKRRFVAAVLELKR--SGRYDEFV -------------------MT----VRKNQATLTADEKRRFVAAVLELKR--SGRYDEFV -------------------MT----VRKSVAALTPDEKRAFVNAVLELKR--TGVYDRYV -------------------MSNKYRVRKNVLHLTDTEKRDFVRTVLILKE--KGIYDRYI -------------------------MTSADGQKDLQSYMDAVTAMLKLPP--SDRRNWYR AAASAGWSFGQEPAQAATAKYHRLNLQNPAAAPFLESYKKAITVMLQLPP--SDARNWYR SVATVFAGKLTGLSAVAADAAPLRVRRNLHGMKMDDPDLSAYREFVGIMK--GKDQTQAL IAAGLLASGLPGTKAFAQIPS-IPWRRSLQGLAWNDPIIETYRDAVRLLN--ALPASDKF QADGDVSIVVGGPASELGRLPVMVRVRKNANQLTPAERDRFISAMAQINNRGTGRFTDFR DDTLLWYSKAVESMKQKDITDPSSWWYQGAIHGYGLDKRPNLANNESWSE--SSVWEQAE SKIAMALFAAGLAFNVSAADIKVAVASDATSLDPQEQLSGQTLEMSHLVFDPLMRYTQDL SAAGL-ASK+GGPAAVAA+MTP--+VRKNQA+LTADEKRRFVAA+LELKR--SGRYDQ+V N-terminal Arginine 29 930 931 932 933 934 935 936 937 938 939 940 941 942 943 944 945 946 947 948 949 950 951 952 953 954 955 956 957 958 959 960 961 962 963 964 965 966 967 968 969 970 971 972 973 974 975 976 977 1 2 4 5 10 16 20 15 19 14 12 17 Cons TTHNAFILGDTDNGE--RTGHRSPSFLPWHRRFLLEFER----ALQSVD--ASVALPYWD TTHNAFIIGDTDAGE--RTGHRSPSFLPWHRRYLLEFER----ALQSVD--ASVALPYWD RTHNEFIMSDTDSGE--RTGHRSPSFLPWHRRFLLDFEQ----ALQSVD--SSVTLPYWD NAHNYYLMSDSDFGP--RIGHRTPSFLPWHRRFLLDFEA----SLQRVD--RNVALPYWD AWHGAAGKFHTPPGSDRNAAHMSSAFLPWHREYLLRFER----DLQSIN--PEVTLPYWE ----------NGFIHLMDCPHGDWWFTSWHRGYLGYFEE----TCRELSGNPDFALPYWD ----------NGFIHTLDCPHGNWWFVVWHRGYTGWFER----TVRELSGDPNFAFPYWD SWLGFANQHGTLNGGYKYCPHGDWYFLPWHRGFVLMYER----AVAALTGYKTFAMPYWN NWVNLSKIHGSGD-VVKYCPHGNWYFLPWHRAYTAMYER----IVRHVTKNNDFAMPFWD NMHVAGRA--------DQQAHGGPGFLPWHRAYLLDLER----ELQAID--PAVTIPYWR GFPPSEGLVNSQFWQ--QCQHGTWFFLPWHRMYLQFFEAIVAKTVVELGGPKDWTLPYWN QFEPRLAEKYERIDDKTVRFHLRKG VKFHSGNDFTADDVVWTVNRLKASPDFKAIFDPI NTHNAFIIGDTDFGE-KRCGHGSPSFLPWHRRYLLDFER----ALQSVDGNPDVALPYWD + Copper A region + + Oxygen-binding motif + + + 1 2 4 5 10 16 20 15 19 14 12 17 Cons WSADRSTR-------------------------------SSLWAPDFLGGTGRSRDGQVM WSADRTAR-------------------------------ASLWAPDFLGGTGRSLDGRVM WSADRTVR-------------------------------ASLWAPDFLGGTGRSTDGRVM WTVDRAAN-------------------------------SPLWASDFMGGSRRGRDGQVL WETDAQMQDPSQ---------------------------SQIWSADFMGGNGNPIKDFIV WTANPEVLPPLFGTILDPVNSSAYIPDHNRFQDIMQEPIKAYWDSLSPAQLQQQNLRGYP WTALPQVPDSFFNGVLDPNNP-AFIASYNEFYSQLSNPMSALWNSFSTAQLQQMRNRGFQ WTEDRLLP------------------------------------EAFT-AKTYNGKTNPL WTDNPYLP------------------------------------EVFTMQKTPDGKDNPL FDRPAPNL----------------------------------FTTDFIGVPDALGTVSFS YCDANNPA---------------------------------------LNPTEQLQALKLP AEAKKVDDFTVDLVTAKPFPLVLQTVT-----------YIFPMDSKFYSGKDEAGKDKAA WTADRQVPDP-F---LDP-N--A-I---N-F------P-SSLWASDFLGGTGRSGDGGVL + 1 2 4 5 10 16 20 15 19 14 12 17 Cons D-GPFAASAGNWP----INVRVDGRTFLRRALGAG--VSELPTRAEVDSVLAMATYDMAP D-GPFAASAGNWP----INVRVDGRAYLRRSLGTA--VRELPTRAEVESVLGMATYDTAP D-GPFAAFTGNWP----INVRVDSRTYLRRSLGGS--VAELPTRAEVESVLAISAYDLPP D-GPFAAGGGKWP----VTVGVDRRDYLRRVLGSG--VPQLPTRAEVDAVLAMPVYDTAP DTGPFAA--GRWTT---IDEQGNPSGGLKRNFGATKEAPTLPTRDDVLNALKITQYDTPP DFDALWSDAMAS-----FANQPNARFLTAQNPKLNPATQTAVDIDTIKASLAPTTFANDA SVNDVWQAVRDSPM---FFPRGRARTLTRQNPGFDATTRRAVSIGTIRNALAPTDFIT-YVPNRNELTGPYAL---TDAIVGQKEVMDKIYAETNFEVFGTSRSVDRSVRPPLVQNSLD YVSSR-TWPITQPM---PDNIVG-PQVLNTILTAKPYEVFGTTR--------PEGQNSLD PANPLQFWATDG-----------VQGILRRQLGASPGAQAAPNILTEAQTLALGSAYR-SEFGTNTPNPDFPG---LWMKERAQYQLSSQADASCSVAMKLQNFTASSPATSFGGVQTG IVKNGDSYASTHVSGTGPFSVKFREQGVKLEYARNANYWDKASKGNVQNLTVVPIKEDAT DVGPFAASAGDWPM---IDVRVDARTYLRRNLGASP-VAELPTRAEVDSVLAPTTYDTAP 30 978 979 980 981 982 983 984 985 986 987 988 989 990 991 992 993 994 995 996 997 998 999 1000 1001 1002 1003 1004 1005 1006 1007 1008 1009 1010 1011 1012 1013 1014 1015 1016 1017 1018 1019 1020 1021 1022 1023 1024 1025 1 2 4 5 10 16 20 15 19 14 12 17 Cons -------WNSGS-DGFRNHLEGWR-GVNLHNRVHVWVGG-----QMATG-VSPNDPVFWL -------WNSAS-DGFRNHLEGWR-GVNLHNRVHVWVGG-----QMATG-MSPNDPVFWL -------YNSAS-EGFRNHLEGWR-GVNLHNRVHVWVGG-----QMATG-VSPNDPVFWL -------WNSSS-SGFRNHLEGWR-GTNLHNRVHVWVGG-----HMATA-ASPNDPVFWL -------WDMTSQNSFRNQLEGFINGPQLHNRVHRWVGG-----QMGVVPTAPNDPVFFL GAPGLAFNSPVSSSHQVAPVGFSILEGQPHNRVHMSVGGQSAPYGLMSQNLSPLDPIFFL ------FGSGKTANHSESAT-QGILESQPHNNVHNNIGG------FMQDLLSPTDPVFFA -----------PKWVPMGGGNQGILERTPHNTVHNNIGA------FMPTAASPRDPVFMM -----------PSWVTTSSGTQGALEYTPHNQVHNNIGG------WMPEMSSPRDPIFFM -------------NFRG-------MQGNPHGSAHVSYFSGS----ISSIPTAAKDPLFFL --------------FSHDSGTFGAVENNPHNLVHVDIGG-----AMGDPNTAALDPIFWL -----------RVAALLGGDVDMIYPVAPNDLERVKNGKD----SQLVTLSGTRAIIIEL ------FWNSASSNGFRNHLEGGILEVNPHNRVHVWVGG-S---QMATGLTSPNDPVFFL + + Copper B region 1 2 4 5 10 16 20 15 19 14 12 17 HHAYIDKLWAEWQRRHPSSPYLPGGGTPNVVDLN----------ETMKPWNDTTPAALLD HNAYVDKLWAEWQRRHPGSGYLPAAGTPDVVDLN----------DRMKPWNDTSPADLLD HHAYVDKLWAEWQRRHPDSAYVPTGGTPDVVDLN----------ETMKPWNTVRPADLLD HHAFIDKLWADWQARNPKAGYLPSGRTQNVIDLR----------GVLPPWNNVTPADMLD HHANVDRIWAVWQIIHRNQNYQPMKNGPFGQNFR----------DPMYPWN-TTPEDVMN HHCNIDRLWDVWTRKQQAMGLPVGPTADQQTQYDPEPYLFYVNADGSPVSDKTRAADYLE HHSNIDRLWDVWTRKQQRLGLPTLPTGANLPLWANEPFLFFIGPDGKPVA-KNKAGDYAT HHGNIDRVWATWNALGRKNSTDPLWLGMKFPNNY---------IDPQGRYYTQGVSDLLS HHCNIDRIWATWN-LRNANSTDRLWADMPFTDNF---------YDVDGNFWSPKVSDLYV LHCNVDRLWAKWQSQVGRYDANVAAAYDAGPTPTSLLAG-HNLHDTLWPWNGIVTPPRPS HHANIDRLWQCWIDQGRENTNDITWLNQVFDFHN-----------ADSLPDTLSVKDVLS NQNTNPALKDKRVRQAINYAINQVGIVDKINKGFG---------TPAGQLSPKGYAGYNE + 1 2 4 5 10 16 20 15 19 14 12 17 Cons HTRH-YTFDV-------------------------------------------------HTAH-YTFDTD------------------------------------------------HTAY-YTFDA-------------------------------------------------HRRF-YTFDKP------------------------------------------------HRKLGYVYDIELRKSKRSS----------------------------------------IGDFDYDYDPGSGEEVIPVATAGRSAPIPALEAAVSASAAVAINKPATAKL--------IGDFDYNYEPGSGEAVIPAASRPGEMNNKVWLGTLGA-AVPNFSASARADV--------TEALGYRY------DVMPRADNKVVNNARAEHLLALFKTGDSVKLADHIRLRSVLKGEHP PEELGYNYGFRTYFKVAAASAKTLALNDKLTSVIAATATDAAIAGVTTTSTDNSKAATEN TAPGGAMAGSSCVSAPGNAPRVSDMLDFQGVVSSSAKLGFAYDDVPLP-----------TEALGFTYSDSYSSSAAPTDSKVFALASAGGSGMFDTIAATTKPFLLGSQSTSAQLEFLP ALKPQYDLAKAKELTKEAGYEKGFKMTFISPAARYVNDVKIAQAVSAMLSKINIKVDLKT HEALGYTYDKGSGEAVAPAASKGFALNFKA-SA-AAA-AAAAIAVPATASL-N-K----P + +Tyrosine motif 31 1026 1027 1028 1029 1030 1031 1032 1033 1034 1035 1036 1037 1038 1039 1040 1041 1042 1043 1044 1045 1046 1047 1048 1049 1050 1051 1052 1053 1054 1055 1056 1057 1058 1059 1060 1061 1062 1063 1064 1065 1066 1067 1068 1069 1 2 4 5 10 16 20 15 19 14 12 17 ---------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------TVSQELVDVAAKPSEQSRQFAKVSIAPPMDVGGL --------------------------MVPEAVPEAAMK-ADGPAVFAKITIAPPMDVAGV V---------ATAVEPLNSAVQFEAGTVTGALG-ADVGTGSTTEVVALIKNIRIP-YNVI VPLSLPIKIPAGALQEIVRQPPLPSGMDTMDFGAAQEQAASAPRVLAFLRDVEITSASTT -----------------------------------------------------------E------------------------KQRAAQVPVLGASNSQTPNQVIIVLDNVTGSGVVA MP--------------------------VAQYWPEFDKCASDMQLIGWHSDTEDSANFFE 1 2 4 5 10 16 20 15 19 14 12 17 -------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------NFLVFISPEGTTPDLNPDGPDFAGSFEFFG--VRHHHTDTVSFTIPIDKALDRLIDDGRL EFHVLVNPPENVSHVDFDSPSFAGTFSVFGKQLGGHKNQPLSFLMPLTEAVKKLQETNEL SIRVFVNLPNANLDVPETDPHFVTSLSFLTHAAGHDHHALPSTMVNLTDTLKALN---IR SVRVFLGKNDLKADTPVTDPHYVGSFAVLGHDG--DHHRKPSFVLDLTDAIQRVYGGRGQ -----------------------------------------------------------PVSVYVKASANSERVLVGKIGLFGLTQSSTPSSTSCLEQGISIELDVTDALQQLRSQTNW FLTFTKDAKTGMGQYNCGGYANAEADKMVMEANTETDPAKRAAILQKVEAMLIDDAAYVP 1 2 4 5 10 16 20 15 19 14 12 17 ------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------KAGEPIDFAVVVAQEGKRVEGSMPAKAQLTDIQVGSF KPGQPLRVQVVAERKGVNLT---PLQAKVSEISVGTF DDNFSINLVAVPQPGVAVESSGGVTPESIEVAVI--TDGEAIDLQLIP-VGSGAGKPGAVEPAKLEIAIVSA------------------------------------NLENLQIELEPGRELGNASVTVGRVSIKAEVV----LHWEDLAYGAKKNVDIKPVVNVMNFPYLGDLVVSK-- Identifier Organism Enzyme Sequence Reference 1 Streptomyces antibioticus TYR NCBI Protein ID: AAA88571.1 [181] 2 Streptomyces glaucescens TYR NCBI Protein ID: AAA26834.1 [30, 45, 149] 3 Streptomyces nigrifaciens TYR na [25] 4 Streptomyces castaneoglobisporus TYR NCBI Protein ID:AAP33665.1 [46] 5 Streptomyces lavendulae TYR NCBI Protein ID:ABQ41256.1 [183] 6 Streptomyces michiganensis TYR na [70 150] 32 1070 7 Streptomyces sp. KY-453 TYR na [183] 8 Streptomyces albus TYR na [47] 9 Streptomyes sp. REN-21 TYR na [59, 74] 10 Bacillus megaterium TYR NCBI Protein ID:ACC86108.1 [6] 11 Bacillus thuringiensis TYR na [26] 12 Marinomonas mediterranea TYR NCBI Protein ID:AAV49996.1 [42, 185] 13 Pseudomonas putida F6 TYR na [186] 14 Ralstonia solanacearum TYR NCBI Protein ID:NP_518458 [43] 15 Ralstonia solanacearum TYR NCBI Protein ID:NP_519622 [43] 16 Sinorhizobium meliloti TYR na [49] 17 Aeromonas media TYR NCBI Protein ID:ACD40043.1 [50] 18 Pseudomonas sp. DSM13540 TYR na [81] 19 Rhizobium etli CFN42 TYR NCBI Protein ID: AAM54973.1 [51, 184] 20 Verrucomicrobium spinosum TYR NCBI Protein ID:ZP_02925214.1 [21] 21 Thermomicrobium roseum TYR na [44] Abbreviations: na, not available 1071 1072 Table 1 Three-dimensional structures of bacterial tyrosinases and features. Organism Conditions Streptomyces castaneoglobisporus 1. Copper-free Caddie protein Yes 2. Copper-bound 3. oxy-form Yes Yes 4. met-form Yes 5. deoxy-form Yes 6. Copper-bound form Yes (deoxy-form, crystal soaked in O2saturated solution) 7. Different copper Yes occupancy 33 PDB Identifier PDB ID:1WX5 PDB ID: 1WXC PDB ID:3AWU PDB ID: 1WX2 PDB ID: 1WX4 PDB ID:2AHK PDB ID:2ZMY PDB ID:2ZMX PDB ID:2AHL PDB ID: 2ZMZ PDB ID:2ZWD PDB ID: 2ZWE PDB ID: 2ZWF PDB ID: 2ZWG PDB ID: 3AWS PDB ID: 3AWV (low) Resolution Reference (Å) 2.02 [5, 147] 1.20 1.16 1.80 1.50 1.71 1.45 1.33 1.60 1.37 1.35 1.32 1.40 1.32 1.24 1.40 1.35 8. Bacillus megaterium 1. 2. 3. 4. 5. PDB ID: 3AWT PDB ID: 3AWW (high) With mutant caddie Yes (H82Q) PDB ID: 3AWX Yes (M84L) PDB ID: 3AWY Yes (H97Q) PDB ID: 3AWZ Yes (Y98F) PDB ID: 3AX0 Copper-bound No PDB ID: 3NM8 PDB ID: 3NPY Copper-bound No PDB ID: 3NQ0 (absence of zinc) (only CuA occupied) PDB ID: 3NTM (CuB partially occupied) Mutant form R209H No PDB ID: 3NQ5 With the inhibitor No PDB ID: 3NQ1 kojic acid In presence of SDS No PDB ID: 4D87 1073 34 1.35 1.25 1.58 1.43 1.40 2.00 2.19 2.20 2.30 2.30 2.30 3.50 [6,60] 1074 1075 Table 2 Substrate specificity of some bacterial tyrosinases. Organism Monophenols Diphenols Polyphenols Reference Bacillus megaterium L/D-tyrosine, tyramine L/D-DOPA, caffeic acid, catechins, catechol, chlorogenic acid pyrogallol, phloroglucinol [36, 48] Bacillus thuringiensis L-tyrosine catechol, L-DOPA, 4-methyl catechol, dopamine, 3,4diihydroxymandelic acid, hydroquinone, 3,4-dihydroxyphenylacetic acid, resorcinol nr [26] Rhizobium etli L-tyrosine, nacetyl-Ltyrosine L-DOPA, catechol, caffeic acid nr [51] Streptomyces antibioticus L-tyrosine p-aminophenol, 2/3-Cl-phenol, tert-butylcathechol, dopamine, L-DOPA, hydro-quinone, p-cresol, p-nitrophenol, dopamine, adrenalin, noradrenalin, 4methyl-catechol, 4nitrocatechol nr [19, 40, 101, 148] Verrucomicrobium spinosum L-tyrosine L-DOPA Sodium caseinate, proteinsa [21, 62] 1076 1077 1078 35 1079 Table 3 Inducible bacterial tyrosinases. 1080 Organism Bacillus megaterium Bacillus thuringiensis Pseudomonas sp. DSM13540 Streptomyces antibioticus Streptomyces castaneoglobisporus Streptomyces glaucescens Streptomyces michiganensis Vibrio cholera Abbreviations: nr, not reported. Enzyme TYR TYR TYR TYR TYR TYR TYR nr Inducing conditions Tyrosine and copper Heat (42°C) L-tyrosine, wool fibres L-methionine Methionine, copper L-tyrosine, L-methionine Copper Osmotic stress, heat (>30°C) 1081 1082 36 Reference [48] [26] [81] [148] [63] [149] [150] [151] 1083 Table 4 Representative applications of tyrosinase in different fields. Field Production of dyes Cosmetic applications Biosensors Biosynthesis and medical applications Bioremediatio n of wastewaters Materials Food applications Others Mode of action Reference 1. Oxidation of an aromatic phenolic compound by tyrosinase and production of coloured compounds 1. Self-tanning agent [97,152, 153] 1. As single enzymes 2. In a coupled assay to detect catechol-producing compounds, e.g. hormones, salicylate 1. Production of L-DOPA 2. Production of substituted catechols 3. Production of food additives 4. Production of estrogenic compounds 5. Production of melanins for therapeutic uses 6. Treatment of neurological diseases 7. Production of mosquito repellants 8. Assay of cholesterol on the skin [155] [156-160] 1. Removal of phenolic and substituted phenolic contaminants 2. Reduction of COD 3. Decolourisation (degradation of dyes) 1. Production of polyphenolic polymers 2. Modifications of materials 3. Production of adhesives 4. Film production 5. Treatment of textiles [119-122, 163-167] 1. Production of tea [178, 179] 2. Production of dairy products [86, 180] 3. Cross-linking of meat proteins 1. As a reporter protein for counting microorganisms 2. Identification of bacteria producing L-tyrosine [143] [181] [154] [55, 87, 88, 91, 94, 95] [106, 111] [113, 114] [112] [115, 161] [116] [162] [118] [168] [126, 127, 169-172] [173] [125, 134-137, 174] [85] [175] [134-137, 176, 177] [34, 35] 1084 1085 37