Synthesis of 4′-thiosemicarbazonegriseofulvin and its
advertisement

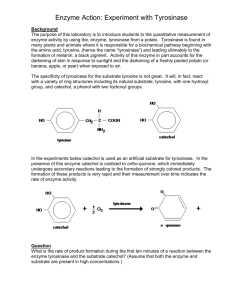
Synthesis of 4′-thiosemicarbazonegriseofulvin and its effects on the control of enzymatic browning and postharvest disease of fruits Zhi-Zhen Pan a# , Yu-Jing Zhua#, Xiao-Jie Yub, Qi-Fan Linb, Rong-feng Xiaoa, Jian-Yang Tanga, Qing-xi Chenb,* and Liu Boa,* a Agricultural Bio-Resources Institute, Fujian Academy of Agricultural Sciences, Fuzhou 350003, China b Key Laboratory of the Ministry of Education for Coastal and Wetland Ecosystems, College of Environment and Ecology, School of Life Science, Xiamen University, Xiamen 361005, China # The authors contribute equally in this work. * Corresponding author: Tel/Fax: +86 591 87864601. E-mail: liubofaas@163.com (B. Liu), chenqx@xmu.edu.cn (Q.-X. Chen). 1 ABSTRACT Browning and postharvest disease of fruits lead to large losses of food industry. A new thiosemicarbazide derivative of griseofulvin (4′-thiosemicarbazonegriseofulvin) was synthesized and evaluated for its potential on the control of browning and postharvest disease of fruits. Browning is mainly due to the enzymatic oxidation of phenolic compounds catalyzed by tyrosinase, 4′-thiosemicarbazonegriseofulvin can effectively inhibit the activity of tyrosinase and its IC50 value was 37.8 μmol/L. It was a reversible and noncompetitive inhibitor of tyrosinase and its inhibition constant (KI) was determined to be 38.42 μmol/L. The antifungal activity of 4′-thiosemicarbazonegriseofulvin was studied on four fungi (F. oxysporum, F. moliniformin, F. solani and Colletotrichum) which often caused postharvest disease of fruits. The results showed the 4′-thiosemicarbazonegriseofulvin can also strongly inhibited the mycelial growth of four target fungi, the LC50 values were 5.4, 7.0, 15.3 and 1.5 mmol/L, respectively. Keywords: 4′-thiosemicarbazonegriseofulvin, browning, postharvest disease 2 1. Introduction Browning and postharvest disease of fruits cause considerable losses to the food industry (Segovia-Bravo, Jaren-Galan, Garcia-Garcia & Garrido-Fernandez, 2007; Wan, Tian & Qin, 2003). The mechanical damage and collision during fruit post-harvest handing and processing will accelerate the browning (Arias, Gonzalez, Oria & Lopez-Buesa, 2007). It reduces not only the visual quality but also results in undesirable changes in flavour and nutrient loss. The browning is mainly due to the enzymatic oxidation of phenolic compounds catalyzed by tyrosinase (EC 1.14.18.1) (Yan et al., 2009). Tyrosinase can catalyze monophenols into o-diphenols and o-diphenols into quinones, respectively (Qiu et al., 2009; Song, Chen, Wang, Qiu & Huang, 2005). Quinones will be converted into brown pigments undergo further non-enzymatic polymerization (Huang et al., 2009). Thus, the tyrosinase inhibitors will be effective antibrowning agents, such as Enokitake mushroom extracts (Jang, Sanada, Ushio, Tanaka & Ohshima, 2002), Artocarpus heterophyllus extracts (Zheng, Cheng, To, Li & Wang, 2008), oxyresveratrol and Morus alba L, extract (Li, Cheng, Cho, He & Wang, 2007). Fruit is easily attacked by several fungal pathogens for the high amount of nutrients, water content and low pH and fruit have lost most intrinsic resistance to the fungal pathogens after harvest, therefor postharvest disease of fruit caused great losses to the food industry (Janisiewicz & Korsten, 2002; Nunes, 2011). Adequate control of postharvest diseases is a prerequisite for the production of a stable and profitable food supply. The pathogens that cause the most important postharvest disease are several species belonging to Alternaria, Aspergillus, Botrytis, Fusarium, Geotrichum, Gloeosporium, Mucor, Monillinia, Penicillium, Colletotrichum and other genera (Kobiler, Akerman, Huberman & Prusky, 2011; Kong, Shan, Liu, Wang & Yu, 2010; Schirra, D'Aquino, Cabras & Angioni, 2011; Sharma, Singh & Singh, 2009). Griseofulvin is one of the representative antifungal antibiotics and has been widely used as an antifungal drug. Its antifungal activity has been demonstrated in many filamentous fungi (Cole & Stricklin, 1989; Gupta, Williams, Zaman & Singh, 2009; Robinson & Robinson, 1959). However, it has weak effect on antibrowning of fruit. Our objective in the present study was to prepare a new thiosemicarbazide derivative of griseofulvin (4′-thiosemicarbazonegriseofulvin) and investigate its inhibitory effects on tyrosinase and antifungal properties. 3 2. Materials and methods 2.1 Reagents Griseofulvin (99.9% powder) was purchased from Shanghai Pharmaceuticals Holding Co., Ltd. (Shanghai, China). Mushroom tyrosinase (EC1.14.18.1) with 6680 U/mg was purchased from Sigma-Aldrich (St. Louis, MO). Dimethyl sulfoxide (DMSO) and L-3,4-dihydroxyphenylalanine (L-DOPA) were obtained from Aldrich (St. Louis, MO). Fusarium oxysporum (FJAT-3701), Fusarium moliniformin (FJAT-165), Fusarium solani (FJAT-176) and Colletotrichum (FJAT-763) were from Fujian Academy of Agricultural Sciences. All other reagents were local products of analytical grade. 2.2 Synthesis of 4′-thiosemicarbazonegriseofulvin The 4′-thiosemicarbazonegriseofulvin was prepared by one-step reaction of griseofulvin and thiosemicarbazide in an acidic solution of ethanol, as previous described (Zhu et al., 2009). Griseofulvin (10 mmol) and acetic acid (10 ml) were added to a solution of thiosemicarbazide (10 mmol) in methanol (60 ml). The mixture was stirred and refluxed for 72 h and cooled to room temperature. Then the reaction mixture was kept under 4 °C for night and filtrated. The product was purified by recrystallized in ethanol. The structure of the product was established by spectroscopic methods (MS and NMR). 2.3 Enzymatic activity assay The enzyme activity assay was performed as reported by (Guo et al., 2010). In this investigation, L-DOPA was used as a substrate for the activity assay of tyrosinase. The reaction media (3 ml) for activity assay contained 0.5 mmol/L L-DOPA in 50 mmol/L sodium phosphate buffer (pH 6.8). The 4′-thiosemicarbazonegriseofulvin were dissolved in DMSO and diluted to appropriate concentrations. The final concentration of DMSO in the test solution was 3.3%. The controls, without inhibitors but containing 3.3% DMSO in the reaction media, were routinely carried out. The reaction was carried out at a constant temperature of 30℃. The inhibition type was assayed by the Lineweaver–Burk plot, and the inhibition constant was determined by the second plots of the apparent Km/Vm or 1/Vm versus the concentration of the 4 inhibitor. A Beckman UV-650 spectrophotometer was used for absorbance and kinetic parameters. 2.4 Antifungal activity assay The antifungal of 4′-thiosemicarbazonegriseofulvin was tested on four phytoathogenic species fungi: F. oxysporum, F. moliniformin, F. solani and Colletotrichum. Some proper concentrations of 4′-thiosemicarbazonegriseofulvin in DMSO were evenly spread on the culture medium PDA, in sterilized Petri dishes and dried for 2 h under a laminar flow, and then F. oxysporum, F. moliniformin, F. solani and Colletotrichum were inoculated in the middle with 100 μl fungal conidial suspension (~1×105 CFU/ml). In parallel, control experiments only with DMSO were also performed. The Petri dishes were incubated at 28±1 °C and the fungal colony diameter was measured daily. The inhibition rates of 4′-thiosemicarbazonegriseofulvin were calculated after 7 days of incubation when mycelium in the control experiments completely covered the dishes. It was expressed as an average diameter and calculated using the following equation: Where Dc and Dt represent mycelium growth diameter in control and treated Petri plates, respectively. The antifungal effects were measured with three replications. Lethal concentration 50 (LC50) values on the target fungi were analyzed using Probit Analysis and considered significantly different if 95% confidence intervals did not overlap. The statistical analyses were done using the China-DPS program. 2.5 Effect of 4′-thiosemicarbazonegriseofulvin on hyphal morphology The effects of 4′-thiosemicarbazonegriseofulvin on hyphal growth of F. oxysporum, F. moliniformin, F. solani and Colletotrichum were studied by using microscope. Ten milliliters of agar medium inoculated with fresh fungal culture (~1×105 CFU/ml) were poured into Petri dishes as the upper layer. After solidification, two wells of 8-mm diameter were created. 16 mmol/L 4′-thiosemicarbazonegriseofulvin solution and controlled DMSO were deposited in wells. Petri dishes were then incubated at 28±1 °C for 72 h. The hyphae closely around the inhibition zones were collected and observed by Leica DMI 3000 M Inverted Microscope (Leica Corp.). 5 3 Results and discussion 3.1 Synthesis of 4′-thiosemicarbazonegriseofulvin The 4′-thiosemicarbazonegriseofulvin (the structure was showed in Fig. 1) was obtained as a white powder. Yield: 85.4%. 1H NMR (CDCl3, 600 MHz): δ (ppm) 7.25 (HN, 1H, s), 5.61 (CH, 2/3H, s), 5.72 (CH, 1/3H, s), 6.12 (bezene, H, s), 6.35, 8.68 (NH2, 4/3 H, s), 6.32, 8.97 (NH2, 2/3 H, s), 3.98, 4.06 (CH3O-bezene, 6H, s), 3.60 (3′-CH3O, 2H, s), 3.67(3′-CH3O, H, s), 2.72(CH, 2/3H, m), 3.10 (CH, 1/3H, m), 1.26 (CH2, 2H, m), 0.96 (CH3, 3H, m). ESI-MS: m/z 426.2 (M+H+). 3.2 Effect of 4′-thiosemicarbazonegriseofulvin on the activity of mushroom tyrosinase We studied the effect of 4′-thiosemicarbazonegriseofulvin on the activity of mushroom tyrosinase using L-DOPA as substrate. The enzyme activity was monitored by following the increasing absorbance at 475 nm accompanying the oxidation of L-DOPA. From the Figure 2, we found 4′-thiosemicarbazonegriseofulvin could effectively inhibit the activity of tyrosinase with dose-dependence. When the concentration of was enhanced to 20 μmol/L, the relative activity of tyrosinase was only to be 64%. The 50% inhibitory concentration (IC50) of 4′-thiosemicarbazonegriseofulvin against tyrosinase was estimated to be 37.8 μmol/L, It was much better than arbutin (IC50=5.32) as previous report (Song, Qiu, Huang & Chen, 2003), which was well-known tyrosinase inhibitors. 3.3 The inhibition mechanism of 4′-thiosemicarbazonegriseofulvin on the activity of mushroom tyrosinase The inhibition mechanism of 4′-thiosemicarbazonegriseofulvin on tyrosinase was investigated and the result was showed in Fig. 3. The plots of enzyme activity versus the concentrations of enzyme in the present of different concentrations of 4′-thiosemicarbazonegriseofulvin gave a family of straight lines, which all passed through the origin indicating that the inhibition of 4′-thiosemicarbazonegriseofulvin on tyrosinase was a reversible reaction course. The present of 4′-thiosemicarbazonegriseofulvin did not bring down the amount of the efficient enzyme, but just resulted in the descending of the activity of the enzyme. 6 3.4 The inhibition type of 4′-thiosemicarbazonegriseofulvin on the activity of mushroom tyrosinase. The inhibitory type of 4′-thiosemicarbazonegriseofulvin on tyrosinase was determined by Michaelis-Menten kinetics. As Fig. 4a showed, the plots of 1/ν versus 1/[S] gave a family of lines with different slope and intersect one another in the X-axis. It meant 4′-thiosemicarbazonegriseofulvin could decrease the value of Vm but have no effect on the Km of tyrosinase. Above all, we concluded that 4′-thiosemicarbazonegriseofulvin was a noncompetitive inhibitor of tyrosinase, it could bind with both the free tyrosinase and the enzyme-substrate complex, and the equilibrium constants are the same. The equilibrium constant (KI) was obtained from Fig. 4b. The value of KI was 38.42 μmol/L. 3.5 Antifungal activity assay The antifungal activity of 4′-thiosemicarbazonegriseofulvin against four target fungi was evaluated from inhibition rates on the mycelium growth. After 7 days of incubation, the inhibition rates were calculated and the results were reported in Figure 5. The results showed that 4′-thiosemicarbazonegriseofulvin had potential antifungal activity on all target fungi and its effectiveness decreases with the dilution. The effect of 4′-thiosemicarbazonegriseofulvin was most efficient on F. moliniformin and Colletotrichum in the concentration of 0.25 mmol/L with the inhibition rates of 23.0% and 22.4%, respectively. However, in the concentration of 1 mmol/L, 4′-thiosemicarbazonegriseofulvin showed most efficient on Colletotrichum with the inhibition rate of 61.9%, which was similar with the concentrations of 4 mmol/L and 16 mmol/L. We estimated LC50 values of 4′-thiosemicarbazonegriseofulvin on F. oxysporum, F. moliniformin, F. solani and Colletotrichum were 5.4, 7.0, 15.3 and 1.5 mmol/L by Probit analysis. It was similar to griseofulvin. Our findings showed that the coupling with griseofulvin and thiosemicarbazide did not hurt the antifungal activity of griseofulvin. The 4′-thiosemicarbazonegriseofulvin could effectively inhibit the mycelium growth of four target fungi, the inhibitory effect was best on Colletotrichum. 7 3.6 Effect of 4′-thiosemicarbazonegriseofulvin on hyphal morphology The alternation of F. oxysporum, F. moliniformin, F. solani and Colletotrichum hyphae grown on PDA amended with 4′-thiosemicarbazonegriseofulvin was studied under microscope. The hyphae of four target fungi treated with DMSO as control treatment were thick, elongated, even and smooth surfaced, whereas degenerative changes was observed on the hyphal morphology in all 4′-thiosemicarbazonegriseofulvin treatments (as showed in Fig. 6). While treated by 16 mmol/L 4′-thiosemicarbazonegriseofulvin, the growth of four target fungi hyphae were strongly inhibited, as indicated by mycelia sparsity, asymmetry, swelling, curling and twisting. 4. Conclusion Browning and postharvest disease were two main issues of fruit industry. Griseofulvin was a broad-spectrum antifungal drug, it can inhibit fungal mitosis by disrupting the mitotic spindle through interaction with polymerized microtubules (Crackower, 1972). However it had a weak effect on antibrowning of fruit. In this study we coupled a thiosemicarbazide group, which may be the functional group of the inhibitors of tyrosinase, to griseofulvin to enhance its antibrowning activity. It was interesting to find that the new compound (4′-thiosemicarbazonegriseofulvin) could effectively inhibit the activity of tyrosinase with the IC50 value was 37.8 μmol/L, which was much better than arbutin, a well-known tyrosinase inhibitor. It can also strongly inhibited the mycelium growth of four target fungi (F. oxysporum, F. moliniformin, F. solani and Colletotrichum), which can often caused postharvest disease of fruits. Therefor the coupling with griseofulvin and thiosemicarbazide could improve the inhibitory activity against tyrosinase but do not hurt the antifungal activity of griseofulvin. The 4′-thiosemicarbazonegriseofulvin may be served as a novel compound to control both enzymatic browning and postharvest disease of fruits. Acknowledgements The present investigation was supported by the Special Fund for Agro-scientific Rese arch in the Public Interest (200903049), the Fujian Funds for Distinguished Young Sci entists (2009J06010). 8 Figure Legends Figure 1 Chemical structure of 4′-thiosemicarbazonegriseofulvin Figure 2 Effect of 4′-thiosemicarbazonegriseofulvin on the activity of mushroom tyrosinase for the oxidation of L-DOPA. Figure 3 Determination of the inhibitory mechanism of 4′-thiosemicarbazonegriseofulvin on mushroom tyrosinase. The concentrations of 4′-thiosemicarbazonegriseofulvin for curves 0-4 were 0, 20, 40, 60 and 80 mol/L, respectively. Figure 4 Determination of the inhibitory type and inhibition constants of 4′-thiosemicarbazonegriseofulvin on mushroom tyrosinase. (a) Lineweaver-Burk plots for inhibiton of 4′-thiosemicarbazonegriseofulvin on mushroom tyrosinase. The concentrations of 4′-thiosemicarbazonegriseofulvin for curves 0-4 were 0, 20, 40, 60 and 80 mol/L, respectively. (b) represented the secondary plot of Km versus concentration of 4′-thiosemicarbazonegriseofulvin to determine the inhibition constant KI. Figure 5 Effects of 4′-thiosemicarbazonegriseofulvin on mycelium growth of F. oxysporum, F. moliniformin, F. solani and Colletotrichum. The bars on each column show standard error. Figure 6 Micrograph of F. oxysporum, F. moliniformin, F. solani and Colletotrichum treated with 4′-thiosemicarbazonegriseofulvin or control. (C1, C2, C3, C4) Displayed micrograph of F.oxysporum, F.moliniformin, F.solani and Colletotrichum mycelium incubation in controlled DMSO. (T1,T2,T3,T4) Displayed micrograph of F. oxysporum, F. moliniformin, F. solani and Colletotrichum mycelium incubation in 4′-thiosemicarbazonegriseofulvin. 9 Figure 1 10 Figure 2 Relative activity (%) 100 80 60 40 20 0 0 20 40 60 [I] ( M) 80 100 11 Figure 3 Enzyme Activity ( M/min) 120 1 100 80 2 60 3 4 5 40 20 0 0.0 2.0 4.0 6.0 8.0 10.0 [E] ( g/ml) 12 Figure 4 4 0.08 0.05 3 0.06 2 0.04 1 0.04 Intercept 1/v ( M/min) -1 5 0.10 0.03 0.02 0.01 0.02 0.00 0 -1.5 -1.0 -0.5 0.0 0.5 1.0 1.5 2.0 2.5 1/[S] (mM )-1 20 40 60 80 [I] ( M) 13 Fusarium solani Inhibition rate(%) Fusarium oxysporum Inhibition rate(%) Inhibition rate(%) Inhibition rate(%) Figure 5 Fusarium moliniformin Colletotrichum 4′-thiosemicarbazonegriseofulvin (mmol/L) 14 Figure 6 C1 T1 C2 T2 C3 T3 C4 T4 15 References Arias, E., Gonzalez, J., Oria, R., & Lopez-Buesa, P. (2007). Ascorbic acid and 4-hexylresorcinol effects on pear PPO and PPO catalyzed browning reaction. J Food Sci, 72(8), C422-429. Cole, G. W., & Stricklin, G. (1989). A comparison of a new oral antifungal, terbinafine, with griseofulvin as therapy for tinea corporis. Arch Dermatol, 125(11), 1537-1539. Crackower, S. (1972). The effects of griseofulvin on mitosis in Aspergillus nidulans. Canadian Journal of Microbiology, 18(5), 683-687. Guo, Y. J., Pan, Z. Z., Chen, C. Q., Hu, Y. H., Liu, F. J., Shi, Y., Yan, J. H., & Chen, Q. X. (2010). Inhibitory effects of fatty acids on the activity of mushroom tyrosinase. Appl Biochem Biotechnol, 162(6), 1564-1573. Gupta, A. K., Williams, J. V., Zaman, M., & Singh, J. (2009). In vitro pharmacodynamic characteristics of griseofulvin against dermatophyte isolates of Trichophyton tonsurans from tinea capitis patients. Medical Mycology, 47(8), 796-801. Huang, Q. S., Zhu, Y. J., Li, H. L., Zhuang, J. X., Zhang, C. L., Zhou, J. J., Li, W. G., & Chen, Q. X. (2009). Inhibitory effects of methyl trans-cinnamate on mushroom tyrosinase and its antimicrobial activities. J Agric Food Chem, 57(6), 2565-2569. Jang, M. S., Sanada, A., Ushio, H., Tanaka, M., & Ohshima, T. (2002). Inhibitory Effects of"Enokitake"Mushroom Extracts on Polyphenol Oxidase and Prevention of Apple Browning. Lebensmittel-Wissenschaft und-Technologie, 35(8), 697-702. Janisiewicz, W. J., & Korsten, L. (2002). Biological control of postharvest diseases of fruits. Annu Rev Phytopathol, 40, 411-441. Kobiler, I., Akerman, M., Huberman, L., & Prusky, D. (2011). Integration of pre-and postharvest treatments for the control of black spot caused by Alternaria alternata in stored persimmon fruit. Postharvest Biology and Technology, 59(2), 166-171. Kong, Q., Shan, S., Liu, Q., Wang, X., & Yu, F. (2010). Biocontrol of Aspergillus flavus on peanut kernels by use of a strain of marine Bacillus megaterium. International journal of food microbiology, 139(1-2), 31-35. Li, H., Cheng, K. W., Cho, C. H., He, Z., & Wang, M. (2007). Oxyresveratrol as an 16 antibrowning agent for cloudy apple juices and fresh-cut apples. Journal of agricultural and food chemistry, 55(7), 2604-2610. Nunes, C. A. (2011). Biological control of postharvest diseases of fruit. European Journal of Plant Pathology, 1-16. Qiu, L., Chen, Q. H., Zhuang, J. X., Zhong, X., Zhou, J. J., Guo, Y. J., & Chen, Q. X. (2009). Inhibitory effects of [alpha]-cyano-4-hydroxycinnamic acid on the activity of mushroom tyrosinase. Food Chemistry, 112(3), 609-613. Robinson, H. M., Jr., & Robinson, R. C. (1959). Griseofulvin, an antifungal antibiotic. Int Rec Med, 172, 737-742. Schirra, M., D'Aquino, S., Cabras, P., & Angioni, A. (2011). Control of postharvest diseases of fruit by heat and fungicides: efficacy, residue levels, and residue persistence. A review. J Agric Food Chem, 59(16), 8531-8542. Segovia-Bravo, K. A., Jaren-Galan, M., Garcia-Garcia, P., & Garrido-Fernandez, A. (2007). Characterization of polyphenol oxidase from the Manzanilla cultivar (Olea europaea pomiformis) and prevention of browning reactions in bruised olive fruits. J Agric Food Chem, 55(16), 6515-6520. Sharma, R., Singh, D., & Singh, R. (2009). Biological control of postharvest diseases of fruits and vegetables by microbial antagonists: A review. Biological control, 50(3), 205-221. Song, K. K., Chen, Q. X., Wang, Q., Qiu, L., & Huang, H. (2005). Inhibitory effects of 4-vinylbenzaldehyde and 4-vinylbenzoic acid on the activity of mushroom tyrosinase. J Enzyme Inhib Med Chem, 20(3), 239-243. Wan, Y. K., Tian, S. P., & Qin, G. Z. (2003). Enhancement of biocontrol activity of yeasts by adding sodium bicarbonate or ammonium molybdate to control postharvest disease of jujube fruits. Lett Appl Microbiol, 37(3), 249-253. Yan, Q., Cao, R., Yi, W., Chen, Z., Wen, H., Ma, L., & Song, H. (2009). Inhibitory effects of 5-benzylidene barbiturate derivatives on mushroom tyrosinase and their antibacterial activities. Eur J Med Chem, 44(10), 4235-4243. Zheng, Z. P., Cheng, K. W., To, J. T. K., Li, H., & Wang, M. (2008). Isolation of tyrosinase inhibitors from Artocarpus heterophyllus and use of its extract as 17 antibrowning agent. Molecular nutrition & food research, 52(12), 1530-1538. Zhu, Y. J., Song, K. K., Li, Z. C., Pan, Z. Z., Guo, Y. J., Zhou, J. J., Wang, Q., Liu, B., & Chen, Q. X. (2009). Antityrosinase and antimicrobial activities of trans-cinnamaldehyde thiosemicarbazone. J Agric Food Chem, 57(12), 5518-5523. 18