Chemistry 30 - Final Exam Review Package - chem30-wmci
advertisement

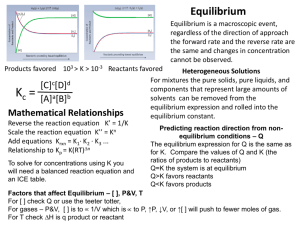
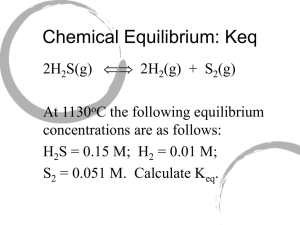
CHEMISTRY 30 REVIEW PACKAGE FOR THE FINAL EXAM Unit 1: Review of Basic Principles Unit 2: Solubility Unit 3: Reaction Rates and Equilibrium Unit 4: Acid/Base Equilibria Unit 5: Oxidation and Reduction (Redox) Unit 6: Molecular Geometry Unit 1: Review of Basic Principles This means know your PSC 20 backward and forward. Types of reactions Naming…You’d better not need to review this by now Mole conversions. Again, you better be able to do these. Using the Activity Series of Metals to determine if a single replacement reaction would proceed. Using a solubility table to determine if a double replacement reaction would proceed. Understanding that if the products of a double replacement reaction were a soluble, liquid, precipitate or gas Making sure to include the states of chemicals in a chemical reaction Stoich Also, know limiting reagents and excess. Trust me, you should know this… Heat in chemical reactions. Endo, exothermic and how to write this in an equation Unit 2: Solubility Understand the term molarity, and be able to calculate any of the three variables from the formula M = moles ÷ litres. Use the formula m1v1 = m2v2 in order to perform dilutions. Determine the concentration of individual ions from a balanced dissociation equation. Understand that only ionic substances dissociate…molecular substances can dissolve, but they do not dissociate. Understand that increased temperature works to increase the solubility of a solid but does the opposite for the solubility of a gas. Be able to interpret a solubility curve and use it to predict precipitation o Super, Un, and plain ol’ saturated Define and describe titration. Perform titration calculations Unit 3: Reaction Rates and Equilibrium Identify factors that affect the rate of a chemical reaction. Understand and explain collision theory. Define the term “activated complex.” Explain how a catalyst works to speed up a reaction. Calculate the rate of a chemical reaction Understand that we can never be sure that a proposed reaction mechanism represents reality: reaction mechanisms are educated guesses! The slow step in a reaction mechanism is the rate determining step. Be able to create, interpret, and label graphs displaying a reaction mechanism Understand and be able to apply Le Chatelier’s Principle: know how changes of pressure, temperature, and concentration affect the dynamics of an equilibrium. When given reaction aA + bB cC + dD, then Keq = [C]c[D]d ÷[A]a[B]b. Remember that only compounds that are gases or are aqueous are included in the Keq expression. Be able to calculate Keq: be able to calculate any other variable in the expression for Keq, given the remaining values. o Remember there are basically 3 ways to calculate concentrations at equilibrium using Keq Understand the concept of Ksp Be able to write a Ksp expression Calculate molar solubility of a saturated solution of a substance when we are given Ksp. Calculate Ksp from molar solubility, or from any expression of concentration Understand the common ion effect Determine whether a precipitate will form by using Ksp – calculate Qsp. Unit 6: Acid-Base Equilibria. State some physical properties of acids and bases Construct an operational definition of an acid and a base, using characteristic properties of those substances Describe the Arrhenius and Bronsted-Lowry conceptual definition of acids and bases Identify the conjugate bases formed in an acid dissociation Identify the conjugate acid of any base Associate the acid or base strength with magnitudes of Ka and Kb Distinguish between strong and weak electrolytes (acids and bases) Recognize substances which are amphiprotic (amphoteric) Write dissociation series for a polyprotic acid Compare the strengths of the dissociation in the dissociation series for a polyprotic acid Write the equation for the ionization of water Write the equilibrium constant expression for the ionization of water Show how the common ion effect influences the equilibrium of water’s ionization when H+ ions or OH- ions are added to water State the value of Kw for pure water Recognize the relationship between [H+] and [OH-] in an aqueous system Calculate the [H+] in solution Express the [H+] as pH, determine [H+] given pH Recognize the relationship between the pH value and the [OH-] Convert values expressed as [H+] and [OH-] to pH and pOH. Determine [OH-] when given pH Recognize the pH at which various indicators undergo a change in colour using an equilibrium reaction. State the general neutralization equation: acid + base salt + water Write equations for neutralization reactions **Titration questions will be in the Unit 2 section** Unit 7: Oxidation and Reduction Define oxidation and reduction in terms of transfer of electrons: LEO goes GER! Know how to assign oxidation numbers to elements in their pure form, and in compounds Be able to distinguish redox reactions from non-redox reactions Balance redox equations (almost everyone needs to review this for sure) Compare reduction potentials for different half-reactions in a table Describe and diagram an electrochemical cell (this covers much detail! Think about all the things you would need to know in order to do this) Calculate the voltage generated by any given electrochemical cell Be able to perform redox stoichiometry calculations Unit 6: Molecular Geometry Be able to draw orbital diagrams and electron configurations for atoms Be able to draw the lewis structure for a molecule Be able to identify: o The Electron group geometry o Molecular geometry o Bond angles o Hybridization around the central atoms (only for sp, sp2, and sp3) o Intermolecular forces experienced o Dominant IMF Be able to discuss the effects of different intramolecular forces on properties (ionic vs. covalent vs. metallic bonding)