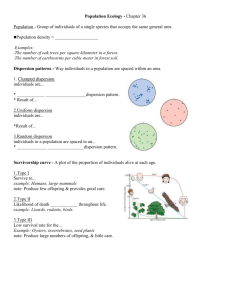
File
advertisement

1999 ANNOTATION (Question B) - Note that they have the same molar, but their solute particle concentrations are different (determine that through the subscript of each atom type). - Freezing point is dependent on solute concentrations. Since MgCl2 has the highest i factor, that will be our answer. ANNOTATION (Question C) - Again, look at solute concentration to determine vapor pressure. - for highest vapor pressure, there must be least solute conc. C2H5OH fits this criteria, so it shall be our answer. GUIDELINES c. 2000 NONE 2001 (A) ANNOTATION a) Since their masses are similar, it cannot be one factor to explain. If you actually read the instructions, it tells you the answer: LOOK AT INTERMOLECULAR FORCES. And so we shall. NH3 has hydrogen bonding between molecules. CH4 has London dispersion (basic). Hydrogen bonding is obviously stronger, so stronger bonds mean harder to break which means higher bp. b) Both of those have London dispersion. SO which one is stronger? C6H14 is larger and therefore more chances to be polar (polar bonds means presence of dipole moments means stronger bond) c) What is special about Si? It is a covalent NETWORK. That must mean something. And it does! It has strong covalent bonds between molecules. Besides, Cl2 only has London dispersion (weakest). Si has stronger bonds which are harder to break therefore higher bp. GUIDELINE 2002 (A) NONE 2002 (B) NONE 2003 (A) QUESTION 8: Only do 8B. OMIT 8A. ANNOTATIONS 6b) i) When they dissolve, salt and sugar becomes IMPURITIES and bond to the water molecules. So it’s harder for water to break free into gas alone. Thus higher bp. That’s one way of thinking. Another (the one AP wants) is that bp depends on vapor pressure above the solution to that above pure water. Salt & sugar are solutes that do not vaporize with water. Their vapor pressure above is lower (harder for water to vaporize) and therefore higher boiling point. 6d) There is water vapor in the air. When they touch the surface of the beaker that is colder, they pass their heat to the beaker and loses heat and CONDENSES ON THE SURFACE of the beaker wall. You must state about EQULIBRIUM vapor pressure for water at lower temp. is lower than pressure that water vapor exerts in the room. 8b) i) Propane only have London dispersion. Propanone has London dispersion and dipole-dipole (stronger). They have different intermolecular forces strengths, so they must have different H(vap). ii) 1-propanol has London dispersion and hydrogen bonding. Propanone only has London dispersion and dipole dipole. Again, different IMF, different H(vap). GUIDELINES 8. 2003 (B) NONE 2004 (A) CHOOSE ONLY 7a AND 7d ANNOTATION: 7a) First, look at polarity. Both are nonpolar. Second, look at IMFs. Ok, both only have London dispersion forces. So you can’t apply my chapter to answer. MUHAHAHAHAH. (but you can use my chapter to get half points!) I2 is larger so more able to be polar than F2, so dispersion forces are stronger in I2 (Now you get to use my section and DERIVE the second half of your points!)—yes, they have dispersion forces, but which is stronger is the question. 7d) NH3 has hydrogen bonding. Phosphine only has dipole dipole or dispersion. Water the solvent has hydrogen bonding. Since it is the same as NH3 in terms of IMF, these pairs are more compatible. NH3 molecules can hydrogen bond with H2O, while Phosphine disadvantage on this side. GUIDELINES 2004 (B) NONE 2005 (A) Question 7. ANNOTATION: 7a) i) NH3 has H. So its London dispersion (basic one) and hydrogen bonding. SIMPLE. NH3 has London dispersion. It’s not nonpolar so it has dipole dipole too. EASY. ii) NH3 has hydrogen bonding, the strongest. So it’s harder to break the bonds so higher bp. EASY. GUIDELINES 2005 (B) Question 8. ANNOTATION: 8d) Propane: dispersion (default) Methanoic acid: Hydrogen bonding and dispersion. e) Methanoic has stronger IMF (hydrogen bonding), needs more energy to break bonds, higher bp. GUIDELINES