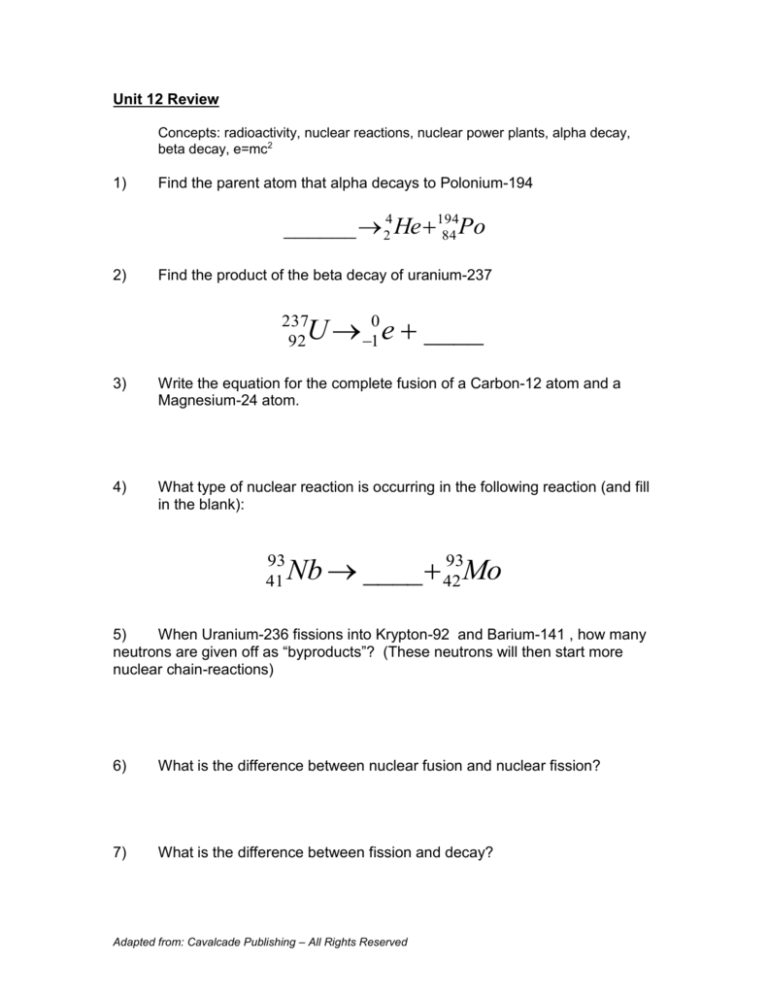
Unit 12 Review
Concepts: radioactivity, nuclear reactions, nuclear power plants, alpha decay,
beta decay, e=mc2
1)
Find the parent atom that alpha decays to Polonium-194
______ 24 He 194
84 Po
2)
Find the product of the beta decay of uranium-237
U 10 e ____
237
92
3)
Write the equation for the complete fusion of a Carbon-12 atom and a
Magnesium-24 atom.
4)
What type of nuclear reaction is occurring in the following reaction (and fill
in the blank):
93
41
93
Nb ____ 42
Mo
5)
When Uranium-236 fissions into Krypton-92 and Barium-141 , how many
neutrons are given off as “byproducts”? (These neutrons will then start more
nuclear chain-reactions)
6)
What is the difference between nuclear fusion and nuclear fission?
7)
What is the difference between fission and decay?
Adapted from: Cavalcade Publishing – All Rights Reserved
Nuclear Chemistry Worksheet – Solutions
Using your knowledge of nuclear chemistry, write the equations for the following
processes:
1)
The alpha decay of radon-198
198
86
2)
Rn 24 He 194
84 Po
The beta decay of uranium-237
U 10 e 237
93 Np
237
92
3)
They fuse into Argon-36
4)
Beta decay! (see #2 for the way to write a beta particle)
5) An induced fission reaction. A neutron is absorbed by a uranium-235 nucleus,
turning it briefly into an excited uranium-236 nucleus, with the excitation energy
provided by the kinetic energy of the neutron plus the forces that bind the neutron.
The uranium-236, in turn, splits into fast-moving lighter elements (fission products)
and releases three free neutrons. At the same time, one or more "prompt gamma
rays" (not shown) are produced, as well. (from Wikipedia)
6)
What is the difference between nuclear fusion and
nuclear fission?
In nuclear fusion, small nuclei are combined to
form a larger nucleus – this process releases a
very large amount of energy, and is the main
source of energy in the sun. In nuclear fission,
large nuclei break apart to form smaller ones,
releasing a large amount of energy. Fission is
used in nuclear power plants to generate
energy.
7)
What is the difference between fission and decay?
In nuclear fission, large nuclei break apart to form (usually) 2 smaller
ones that are relatively equal size, releasing a large amount of
energy. In decay, a large nucleus breaks into a small particle (alpha,
beta, etc) and an almost as large nucleus.
Adapted from: Cavalcade Publishing – All Rights Reserved










