Colligative Properties
advertisement

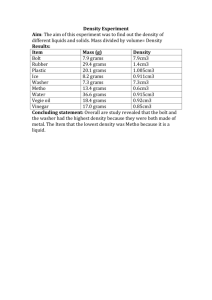
Name ______________________________________________ Colligative Properties Molality (m)= moles of solute/ kilogram of solvent The molal freezing point constant for water (Kf) = -1.86 °C/m The molal boiling point constant for water (Kb)= 0.51 °C/m ΔTf = Kf × m × i ΔTb = Kb × m × i i = number of moles of ions produced per mole of solute 1. If one mole of NaOH is dissolved in 1000 grams of water, what is the molality of this solution? 2. What is the molality of a solution that has 40 grams of NaOH dissolved in 1.00 kilogram of water? 3. If 80.0 grams of NaOH is dissolved in 1 kg of water, what is the molality of this solution? 4. What is the molality of a solution that has 58.45 grams of NaCl dissolved in 500.0 grams of water. 5. A solution was prepared by dissolving 342.24 grams of table sugar (sucrose), C12H22O11, in 2000 grams of water. Find the molal concentration of this solution. 6. If 29.23 grams of table salt (NaCl) is dissolved in 500 grams of water, what is the molality of this solution? 7. What is the boiling point of a solution made by dissolving 1.0000 mole of sucrose in 1.0000 kilogram of water? 8. What is the freezing point of a solution made by dissolving 342.2 grams of table sugar (C12H22O11) in 1000.0 grams of water? 9. A solution is made by dissolving 58.45 grams of salt in 1000.0 grams of water. At what temperature will this solution freeze? 10. At what temperature will the solution in # 9 above boil? 11. What is the boiling point of a calcium fluoride solution when 200.00 grams of CaF 2 is dissolved in 752 grams of water? Freezing point? 12. How many grams of sodium chloride must be dissolved in 3000.0 grams of water so that it will boil at 105 °C? 13. How many grams of sodium chloride must be dissolved in 3000.0 grams of water so that the solution freezes at -5.00 °C? 14. Which will have the lowest freezing point—50.0 grams of sucrose in 1000.0 grams of water or 25.0 grams of NaCl in 1000.0 grams of water?