Unit II, Fundamentals - LSU Fire and Emergency Training Institute
advertisement

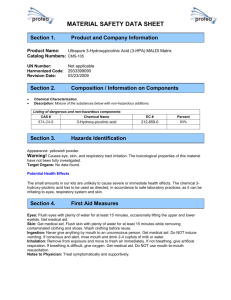
Emergency Response to Terrorism: Tactical Considerations: Hazardous Materials Student Manual Unit 5: Protection Terminal Objective Given an exercise, the students will select appropriate personal protective equipment (PPE) based on the chemical and physical properties of the agent. Enabling Objectives The students will: Identify the types and importance of respiratory protection relative to a terrorist event. Identify the advantages and risks involved with using conventional PPE in a terrorist response. EMERGENCY RESPONSE TO TERRORISM: TACTICAL CONSIDERATIONS: HAZARDOUS MATERIALS SM 5-2 UNIT 5: PROTECTION INTRODUCTION As stated in Unit 1 Introduction, the focus of this training is protection and detection. One of the key factors when responding to an incident involving a biological, nuclear, chemical, or etiological agent is selecting the proper level of protection for the incident. In the early days of hazardous material response, there was a tendency to dress in Level A protection without doing a complete risk assessment. A haz mat team would respond to two drums of an unidentified product and would always dress in Level A, yet our counterparts in the Environmental Protection Agency (EPA) would respond to a hazardous waste site with hundreds of drums on the site and would dress in Level C. This same mentality is being applied to the Biological, Nuclear, Incendiary, Chemical, and Explosive (B-NICE) arena of chemicals. These chemicals are being produced by scientists working for various governments, with the intent to kill hundreds of troops. Even with this knowledge, the military would respond in mission-oriented protective (MOP) gear (Level C). Today, some militant group mixes something in their basement and we, the responders, jump into Level A. This section will provide you with the knowledge to select the appropriate level of protection for the agent involved. THE IMPORTANCE OF RESPIRATORY PROTECTION Respiratory protection is of primary importance since inhalation is one of the major routes of exposure to chemical agents. Prior to discussing types of respiratory protection, we must discuss the hazards that can affect the respiratory system. Respiratory hazards fall into three general categories: airborne contaminants or aerosols; inhalation of gases or vapors; and oxygen-deficient atmospheres. We will examine all three types of hazards and their associated physical and physiological effects. An airborne contaminant or aerosol is a suspension of a liquid or solid particle in a gas. In the ambient air, it can take on various physical forms: Dusts--contain solid particles suspended in the air. Mists--the suspension of liquid particles formed by condensation from vapors or by mechanical actions. Those actions can include splashing of or atomization of the particles. SM 5-3 EMERGENCY RESPONSE TO TERRORISM: TACTICAL CONSIDERATIONS: HAZARDOUS MATERIALS Fumes--suspended solid particles formed when a volatilized solid condenses in cool air. Fiber--suspended particle whose length is at least three times its diameter. Irritating smoke--an aerosol formed from the combustion of organic matter. The second type of respiratory hazard that could be encountered is gases and vapors. A gas is defined as a state of matter that: can be expanded indefinitely; mixes easily into other gases; occupies most containers completely and uniformly; and will expand or contract with temperature and pressures. Vapors differ from gases in that they are normally solids or liquids at standard temperature and pressure. When considering the type of protection required, the type of gases and vapors must be considered. The third type of hazard exists in oxygen-deficient atmospheres. The normal content of oxygen in air at sea level is 21 percent. The Occupational Safety and Health Administration (OSHA) states that an atmosphere that contains 19.5 percent or less is considered immediately dangerous to life and health (IDLH). As the oxygen decreases, physiological effects will take place, and death can occur. Oxygen deficiency can occur either as a result of lack of oxygen in an area, resulting in simple asphyxiation, or because of the presence of chemicals that, through a chemical change, leave the body unable to use oxygen. This condition is called chemical asphyxiation. TYPES OF RESPIRATORY PROTECTION There are three types of respiratory protection: air-purifying respirators (APR's); self-contained breathing apparatus (SCBA); and Supplied-air respirators (SAR's). Air-Purifying Respirators When an incident occurs, according to current thought, tons of SCBA units will be required. The necessity of refilling bottles either on site or SM 5-4 UNIT 5: PROTECTION off site, however, is a serious limitation that must be considered. In reality, the hot zone most likely will be locked down as a crime scene, with perhaps hundreds of people exiting the site, making offsite refilling not a particularly viable option, and limiting the practicality of SCBA's for all responders in such an incident. The protection of responders when dealing with many injuries will require APR's. APR's consist of several components designed to filter out airborne contaminants through the use of filtration, absorption, or chemical reaction. APR's have a role in B-NICE response, but can be used only when the ambient atmosphere contains sufficient oxygen (19.5 percent). They are approved for use in atmospheres containing specific chemicals up to designated concentrations, but not in IDLH atmospheres. Conditions that exclude or may exclude the use of APR's include Oxygen deficiency. IDLH concentrations of specific chemicals. Entry into an unventilated or confined area where the exposure conditions have not been characterized. Presence of or potential presence of unidentified agents. Contaminant concentrations that are unknown or exceed designated maximum use concentration(s). Identified gases or vapors that have inadequate warning properties, when the absorbent's service life is not known and the APR unit has no end-of-service indicator (ESLI). High relative humidity (which may reduce the protection offered by the absorbent). Components of Air-Purifying Respirators full or partial facepiece; filter/absorbent cartridge; and exhalation valve. SM 5-5 EMERGENCY RESPONSE TO TERRORISM: TACTICAL CONSIDERATIONS: HAZARDOUS MATERIALS Advantages lightweight; least expensive; some are disposable; and useful for support operations (e.g., patient decon, EMS, mass decontamination). Limitations become saturated with particles and other contaminants; don't supply oxygen; must have at least 19.5 percent oxygen to use; can't be used with chemicals with poor warning properties; and can't be used in IDLH atmospheres; APR's usually operate only in the negative-pressure mode except for powered air respirators, which maintain a positive pressure to the face piece (except at maximal breathing rates). There are basically three types of filtering devices: particulate filters; cartridges or canisters that contain absorbents for specific gases and vapors; and combination devices. Cartridges usually attach directly to the respirator facepiece. The larger volume canisters attach to the chin of the facepiece or are carried with a harness and attached to the facepiece by a breathing tube. Combination cartridges and canisters contain layers of different absorbent materials and remove multiple chemicals or multiple classes of chemicals from the ambient air. Though approved for use against more than one substance, these canisters and cartridges are tested independently against single substances. Thus, the effectiveness of these canisters against two or more substances has not been demonstrated. Filters also may be provided with cartridges to offer additional protection against particulates. They are color coded to indicate the general chemicals or classes of chemicals against which they are effective. See the Code of Federal Regulations, Title 29 (29 CFR) Part 1910.134. SM 5-6 UNIT 5: PROTECTION Color Coding for Cartridges Contaminants Acid gases Hydrocyanic acid gas Chlorine gas Organic vapors Ammonia gas Acid gases & ammonia gas Carbon monoxide Acid gases & organic vapors Hydrocyanic acid gas & chloropicrin vapor Acid gases, organic vapors, & ammonia gas Radioactive (except tritium & noble gases) Particulates w/any of above All of the above atmospheric contaminants Color assigned White White w/1/2 green stripe White w/1/2 yellow stripe Black Green Green w/1/2 white stripe Blue Yellow Yellow w/1/2 blue stripe Brown Purple (magenta) Canister color w/gray stripe Red w/gray stripe Most chemical-absorbent canisters are imprinted with an expiration date. They may be used up to that date as long as they have not been opened previously. Once opened, they begin to absorb humidity and air contaminants whether or not they are in use. Their efficiency and service life decreases, and therefore they should be used immediately. Cartridges should be discarded after use and should not be used for longer than one incident or when breakthrough occurs, whichever comes first. Where a canister or cartridge is being used against gases or vapors, the appropriate device should be used only if the chemical(s) have "adequate warning properties." The National Institute for Occupational Safety and Health (NIOSH) considers a substance to have adequate warning properties when its odor, taste, or irritant effects are detectable at concentrations below the recommended exposure limit (REL). A substance is considered to have poor warning properties when its odor or irritation threshold is above the applicable exposure limit. Warning properties are essential to the safe use of air purifying respirators since they allow detection of contaminant breakthrough, should it occur. While warning properties are not foolproof (because they rely on human senses which vary widely among individuals and in the same individual under varying conditions, e.g., olfactory fatigue), they do provide some SM 5-7 EMERGENCY RESPONSE TO TERRORISM: TACTICAL CONSIDERATIONS: HAZARDOUS MATERIALS indication of possible absorbent exhaustion, poor facepiece fit, or other malfunctions. Self-Contained Breathing Apparatus A self-contained breathing apparatus (SCBA) usually consists of several components carried by the wearer. Only positive-pressure SCBA's are recommended for entry into IDLH atmospheres. SCBA's offer protection against most types and levels of airborne contaminants. However, the duration of the air supply is an important planning factor in SCBA use. This is limited to the amount of air carried and the consumption rate of the wearer. Also, SCBA's are bulky and heavy, thus they increase the likelihood of heat stress, and may impair movement in confined spaces. Generally, only workers handling hazardous materials or operating in contaminated zones require SCBA's. Entry and escape SCBA's give workers untethered access to nearly all portions of the work site, but may decrease worker mobility, particularly in confined areas, due to both the bulk and weight of the units. Their use is particularly advisable when dealing with unidentified and unqualified airborne contaminants. Types of Self-Contained Breathing Apparatus Open circuit: In the open circuit type, air is exhaled directly into the ambient atmosphere. This is the most widely used type of SCBA. Closed circuit: In the closed circuit type, exhaled air is recycled by removing the carbon dioxide with an alkaline scrubber and replenishing it with oxygen from a solid, liquid, or gaseous source. The advantages of a closed circuit SCBA versus an open circuit are that they can have longer durations of use (30 minutes to 4 hours) and are lighter in weight. On the other hand, it is important to note that with a closed circuit type, the wearer carries a hazardous material on his/her back (oxygen or an oxygengenerating chemical). Also, the chemical reaction that removes the carbon dioxide causes the production of heat. This in turn can increase stress on the wearer, especially in fully encapsulating chemical-protective suits. Positive Pressure The concept of positive pressure means that there is a constant pressure at the facepiece of the SCBA at most times. This is created in most SCBA's by allowing a small amount of air to bypass the regulator (slightly higher SM 5-8 UNIT 5: PROTECTION than atmospheric pressure). If there should be a leak in the face-to-facepiece seal or in the breathing tube, any airborne contaminant would be pushed away from the wearer instead of being inhaled. It is important to note that in some instances the wearer can breathe in a manner that overpowers the positive pressure and causes airborne contaminants to be inhaled through leaks in the system. Advantages of SCBA's: highest level of respiratory protection, wearer has good mobility; and thirty minutes to 4-hour duration, which allows rotation of personnel. Disadvantages: Air supply is limited; therefore work time is limited. Weight of the unit (approximately 30 pounds). Supplied-Air Respirator Supplied-air respirators (SAR's; also known as airline respirators) supply air, never oxygen, to a facepiece via a supply line from a stationary source. SAR's are available in positive- and negative-pressure modes. Pressuredemand SAR's with escape provisions provide the highest level of protection (among SAR's) and are the only SAR's recommended for use at hazardous waste sites. SAR's are not recommended for entry into IDLH atmospheres unless the apparatus is equipped with an escape SCBA. Advantages: offer high level of respiratory protection; can be used for extended periods of time; and lighter than SCBA's and may place less stress on the wearer. Disadvantages: Air must enter and exit via same path. Airline restricts mobility. Maximum hose length of 300 feet. Airline hoses are not tested for chemical resistance. SM 5-9 EMERGENCY RESPONSE TO TERRORISM: TACTICAL CONSIDERATIONS: HAZARDOUS MATERIALS The air source for supplied-air respirators may be compressed air cylinders or a compressor that purifies and delivers air to the facepiece. SAR's suitable for use with compressed air are classified as "Type C" SAR's. All SAR couplings must be incompatible with the outlets of other gas systems used on site to prevent a worker from connecting with an inappropriate compressed gas source (OSHA 29 CFR 1910.134). SAR's enable longer work periods than the SCBA's and are less bulky. However, the airline impairs workers' mobility and requires workers to retrace their steps when leaving the area. Also, the airline is vulnerable to puncture from rough or sharp surfaces, chemical permeation, damage from contact with heavy equipment, and obstruction from falling drums, etc. To the extent possible, all such hazards should be removed prior to use. When in use, airlines should be kept as short as possible (300 feet [100 meters] is the longest approved hose length for SAR's), and other workers and vehicles should be kept away from the airline. The use of air compressors as the air source for an SAR at a hazardous waste site is severely limited by the same concern that requires workers to wear respirators--that is, the questionable quality of the ambient air. Onsite compressor use is limited by OSHA standards (29 CFR Part 1910.134). CONVENTIONAL PERSONAL PROTECTIVE EQUIPMENT In the realm of PPE, responders traditionally have believed that the best protection from agents were the garments that were provided to the military. Recently, many governmental response agencies have been looking at the more conventional garments worn by responders for most chemical incidents. As a review, let's examine the levels of protection as outlined by the EPA. Level A Level A protection is required for entry into areas of very high contamination that pose risks of inhalation, skin, mucous membrane, and eye exposure. This level of protection is required for toxic environments that exceed the IDLH level and for prolonged work at contamination levels greater than the STEL. SM 5-10 UNIT 5: PROTECTION To achieve Level A protection, PPE must include at least: positive-pressure SCBA or positive-pressure air; line with escape options; fully encapsulating chemical-resistant suit; double layer of chemical-resistant gloves; chemical-resistant boots; airtight seals between the suit and the gloves and boots; and communications. Level B Level B equipment provides respiratory protection comparable to that of Level A, but less protection against skin, mucous membrane, and eye exposures. Level B PPE suits are chemical resistant and offer protection against splash exposures. They may be fully or nonencapsulating and allow vapor and dust to enter at the neck, wrists, and closures. Level B protection is the minimum level that should be used for entry into a BNICE site that has not been tested and monitored fully. To achieve Level B protection, PPE must include at least: positive-pressure SCBA; chemical-resistant, long-sleeved suit; double layer of chemical-resistant gloves; chemical-resistant boots; hard hat; and communications. Level C Level C equipment is appropriate for use when the agents have been identified, their concentrations in air are known to fall within the effective range of air-purification filters, and there is little likelihood of exposure of the skin, mucous membranes, or eyes. To achieve Level C protection, PPE must include at least: full-face air-purification device (respirator); chemical-resistant suit; chemical-resistant outer gloves; and chemical-resistant boots. SM 5-11 EMERGENCY RESPONSE TO TERRORISM: TACTICAL CONSIDERATIONS: HAZARDOUS MATERIALS Level D Level D equipment is essentially common work clothes that do not provide specific respiratory or skin protection. This level of PPE should not be worn alone in any environment that poses a hazardous materials risk as a result of respiratory, skin, mucous membrane, or eye exposure. Military Protective Ensembles Individual Protective Equipment A soldier's mission-oriented protection posture (MOPP) gear protects against NBC contamination. It consists of the overgarment, mask, hood, overboots, protective gloves, individual decon kits, detection equipment, and antidotes. Before soldiers can protect themselves against nuclear, biological, and chemical (NBC) hazards, they must first know what individual protective equipment is available and its capabilities. Protective Ensemble Various armies of the world use different types of chemical-protective clothing for individual protection. Several types are available in the U.S. Army. The type depends on the protection required, but all fall within two major divisions: permeable and impermeable. Permeable clothing allows air and moisture to pass through the fabric. Impermeable clothing does not. An example of impermeable clothing is the special butyl rubber suits worn by some explosive ordnance disposal (EOD) soldiers and decon soldiers. Most troops use permeable suits known as battledress overgarments (BDO's). Battle Dress Overgarment Description The BDO is a camouflage-colored (woodland or desert), expendable twopiece overgarment consisting of one coat and one pair of trousers. The jacket has a zipped front, and the trousers have a fly front and zipped legs. The overgarment material consists of an outer layer of nylon/cotton and an inner layer of charcoal-impregnated polyurethane foam. Due to the heavy impregnation of charcoal, some charcoal may be deposited on skin and clothing under the BDO; however, this will not detract from the BDO's chemical-protective characteristics nor harm the wearer. The BDO presently comes sealed in a vapor-barrier bag that protects against rain, SM 5-12 UNIT 5: PROTECTION moisture, and sunlight. The BDO is water resistant, but not waterproof, and normally is worn as an outer garment over the duty uniform; however, in high temperatures it may be worn over underwear. In extreme cold weather environments, the BDO is sized to wear over Arctic/extreme cold weather environmental clothing; however, mission requirements may dictate that the BDO be worn under Arctic clothing. For example, soldiers may need to wear a white Arctic outergarment to help ensure needed cover and concealment. Protection Capabilities The BDO provides protection against chemical agent vapors, liquid droplets, biological agents, toxins, and radioactive alpha and beta particles. When the BDO is removed from its vapor-barrier bag and worn, its protective qualities last for a minimum of 30 days. It is recommended that the BDO be replaced after 30 days; however, the commander may extend the wear time when operationally necessary. BDO's worn longer than 30 days present a slightly increased risk to the wearer; however, the key to BDO effectiveness at any time during wear is its serviceability. Wear time for the BDO begins when it is removed from its sealed vaporbarrier bag, and stops when the BDO is sealed back in its vapor-barrier bag. If the original vapor-barrier bag is not available, return the BDO to a bag of similar material and seal with common duct tape. Donning of the BDO regardless of the length of time equates to a day of wear. Extending the wear time for the BDO affords additional flexibility in operational and logistical support planning. The BDO provides a minimum of 24 hours of protection against exposure to liquid or vapor chemical agent. Exchange the BDO within 24 hours of exposure to a liquid chemical agent. The BDO is not designed to be decontaminated or reimpregnated for reuse. Serviceability The BDO becomes unserviceable if it is ripped or torn, if its fastener is broken or missing, or if petroleum, oils, or lubricants are spilled or splashed on the garment. For example, if a product spills on a BDO sleeve or trouser leg and soaks through the BDO material, replace the BDO. Further, the BDO remains serviceable if the vapor-barrier bag suffers damage (i.e., pinholes, rips, tears), provided the overgarment has not been physically damaged or exposed to water, spills, or chemical agents. When any packaging leaks are discovered, seal/repair them as soon as possible. Common duct tape provides an appropriate and expedient way to repair the vapor-barrier bag. Sealing the bag protects the BDO from direct exposure to moisture, smoke, and fuel solvent vapors which can SM 5-13 EMERGENCY RESPONSE TO TERRORISM: TACTICAL CONSIDERATIONS: HAZARDOUS MATERIALS jeopardize the BDO protective qualities; however, if the original vaporbarrier bag is no longer available to the soldier for overgarment storage, use a replacement storage bag that, as a minimum, is water resistant or water repellent. Chemical Protective Overgarment Description The CPOG is a plain OD green, expendable two-piece overgarment consisting of one coat and a pair of trousers. The jacket has a full-length zipper opening covered by a protective flap. The trousers have a fly front and zipper closure on the lower outside section of each leg. The CPOG is made of material having an outer layer of nylon/cotton and an inner layer of charcoal-impregnated polyurethane foam. Due to the heavy impregnation of charcoal, some charcoal will be deposited on the skin and clothing under the overgarment; however, this will not detract from the chemical-protective characteristics of the suit nor harm the wearer. The CPOG comes sealed in a vapor-barrier bag that protects against rain, moisture, and sunlight. To protect the protective qualities of the CPOG against rain, wet-weather gear should be worn over the overgarment. The CPOG is normally worn over the duty uniform; however, in high temperature it may be worn over underwear. In extreme cold weather, the CPOG is sized to wear over Arctic extreme cold weather environmental clothing; however, mission requirements may dictate that the CPOG be worn under Arctic clothing. For example, soldiers may need to wear a white Arctic outer garment to help ensure needed cover and concealment. Protection Capabilities The CPOG provides protection against chemical agent vapors, liquid droplets, biological agents, toxins, and radioactive alpha and beta particles. When the CPOG is removed from its vapor-barrier bag, its protective qualities last for a minimum of 14 days. It is recommended that the CPOG be replaced after 14 days; however, the commander may extend the wear times when operationally necessary. CPOG's worn longer than 14 days present a slightly increased risk to the wearer; however, the key to CPOG effectiveness at any time during its wear is its serviceability. Wear time for the CPOG begins when it is removed from its sealed vaporbarrier bag, and stops when the CPOG is sealed back in its vapor-barrier bag. If the original vapor-barrier bag is not available, return the CPOG to a bag of a similar (i.e., waterproof) material and seal with common duct tape (for example, double plastic trash bags are a possibility). Donning of SM 5-14 UNIT 5: PROTECTION the CPOG, regardless of the length of time, equates to a day of wear. Extending the wear time for the CPOG affords additional flexibility in operational and logistical support planning. The CPOG provides a minimum of 6 hours of protection against exposure to liquid or vapor chemical agents. Exchange the CPOG within 6 hours of exposure to a liquid chemical agent. The CPOG is not designed to be decontaminated or reimpregnated for reuse. Serviceability The CPOG becomes unserviceable if it is ripped or torn, if its fasteners are broken or missing, or if petroleum, oils, or lubricants (POL) are spilled or splashed on the garment. For example, if a POL spill on a CPOG sleeve or trouser leg soaks through the CPOG material, replace the CPOG. Further, the overgarment remains serviceable if the CPOG vapor-barrier bag suffers damage (i.e., pinholes, rips, tears), provided the overgarment has not been physically damaged or exposed to water, spills, or chemical agents. When any packaging leaks are discovered, seal/repair them as soon as possible. Common duct tape provides an appropriate and expedient way to repair the vapor-barrier bag. Sealing the bag protects the CPOG from direct exposure to moisture, smoke, and fuel solvent vapor which can jeopardize the CPOG's protective qualities; however, if the original vapor-barrier bag is no longer available to the soldier for overgarment storage, use a replacement bag that, at a minimum, is water resistant or water repellent. For example, the waterproof bag can be used for storage. Contamination Avoidance and Liquid Protective Suit The suit, contamination avoidance and liquid-protective (SCALP), is a four-piece suit consisting of jacket, trousers, and two footwear covers. The base cloth material is of high-density polyethylene fibers, and the footwear covers have embossed polyethylene soles for durability and slip resistance. The jacket is a pullover design with an integral hood and covers the head, chest, and arms. An opening is provided for the facepiece of the individual protective mask. Two drawstrings, each with a barrelock, secure the hood to the facepiece, and latex bands secure sleeves around the wrists. The trousers contain a drawstring with a barrelock at the waist and latex bands on the legs to secure them around the ankles. The footwear covers consist of polyethylene soles and latex bands in the upper portion to secure them to the legs. The SCALP jacket/trousers are issued separately from the SCALP footwear covers, since the sizing systems are independent of one another. The SCALP, being a disposable, lightweight, impermeable suit, is to be worn on the outside to keep from SM 5-15 EMERGENCY RESPONSE TO TERRORISM: TACTICAL CONSIDERATIONS: HAZARDOUS MATERIALS gross liquid contamination for periods up to 1 hour. The primary users are armor and EOD personnel and personnel in collective protection who may, by necessity, be forced to leave that collective protection to perform some vital maintenance or reconnaissance function. In such situations, the SCALP also will reduce reentry time. A secondary use of the SCALP is to protect decontamination personnel from being soaked during decontamination operations. Commanders must be aware that wearing the SCALP over the BDO will place an additional burden on the soldier, increasing heat stress problems already associated with wearing the BDO. The SCALP weighs approximately 1.5 pounds. Integrated Battlefield Aircrew Uniform The aircrew uniform, integrated battlefield (AUIB), is a standard combat uniform for aircrews designed to replace the CPOG, BDO, and Nomex flight suit. The AUIB provides NBC protection and protection against flames. It is a two-piece chemical-protective uniform with a protective curtain and stand-up collar. The collar closes with a hook-and-pile tape. The suit has a slide fastener front closure with protective flap and a gusseted fastener leg closure for quick and easy donning and doffing. The wrists and ankles have hook-and-pile adjustments to ensure a tighter fit. Chest pockets are side openings for easy access when the safety harness is in use. Side thigh and calf pockets have bellows on one side for easy access. Insulated pockets for atropine injectors are provided on the upper sleeve. All pockets are lined with butyl rubber. Toxicological Agent Protective Apron The toxicological agent protective (TAP) apron is intended for personnel whose duties may bring them into contact with liquid chemical agents: for example, those who work with toxic munitions, perform decontamination in a field environment, handle contaminated clothing and equipment at a decontamination site, and handle and treat chemical agent casualties. On the battlefield, the TAP apron provides chemical decontamination units with added protection when conducting extended decontamination operations. Chemical Protective Clothing Selection Criteria SM 5-16 Have you identified the agent involved and determined its physical, chemical, and toxicological properties? Does the product have a high vapor pressure? UNIT 5: PROTECTION At the concentrations expected, is the substance a skin hazard? Select the material which provides the least permeation and degradation. Determine whether a fully encapsulating suit is required or if nonencapsulating is sufficient. In those incidents where the presence of the agent is not certain or the agent cannot be readily identified, there usually are clues that can assist in choosing the level of protective clothing. Observations which could indicate that fully encapsulating suits should be worn are visible emissions of gases, vapors, dust, or smoke; indications of airborne hazards on direct-read instruments; configurations of containers or vehicles that indicate they contain gases or pressurized liquids; enclosed, poorly ventilated areas where toxic vapors, gases, and other airborne substances could accumulate; and work functions required that might expose workers to high concentrations of skin toxins. Unknown situations require considerable judgment as to whether maximum protection to the skin (fully encapsulating clothing) is necessary or whether splash suits are appropriate. Heat stress: Nonencapsulating clothing generally causes less heat stress. However, as less area of the body is exposed by wearing gloves and hoods and taping hoods to respirator masks, there is little difference in the heat buildup of either style. AGENT-SPECIFIC PERSONAL PROTECTIVE EQUIPMENT/CHEMICAL PROTECTIVE CLOTHING MATRIX Biological agents enter the body through the respiratory tract, digestive tract, and breaks in the skin. A HEPA filter accompanied by Level C clothing provides adequate protection against all biological agent threats. Radiological agents may enter the body through the respiratory tract, the digestive tract, and breaks in the skin (or in the case of gamma radiation, through the skin). A high-energy air particulate (HEPA) filter accompanied by Level C clothing provides adequate protection against alpha and beta radiation exposure hazards (the respiratory tract, digestive tract, or breaks in the skin). SM 5-17 EMERGENCY RESPONSE TO TERRORISM: TACTICAL CONSIDERATIONS: HAZARDOUS MATERIALS Nerve agents (e.g., Tabun, Sarin, Soman, VX) present both a respiratory and a dermal hazard. In liquid form, droplets may be absorbed by the skin; in a vapor state, they may enter the body through the lungs. Blister agents (e.g., mustard) are designed to cause both internal and external injury. With both a dermal and respiratory threat, protection is required for each route of entry. Choking agents (e.g., phosgene, chlorine) enter the body through the lungs, but may harm the skin; consequently, respiratory protection is the key to protection in a choking agent environment. Choking agents are reasonably nonpersistent, so the level of protection may be downgraded (1) as soon as the concentration in the affected area is determined to be below IDLH and, (2) if the respirator to be used has been proven to protect against the particular choking agent. Blood agents (e.g., hydrogen cyanide, cyanogen chloride) enter the body primarily through membranes and at high levels through the skin. Since blood agents, by definition, are extremely volatile, they will dissipate quickly in the air, probably by the time measurements are taken to determine the concentration of the agent. If the agent vapor concentration is below IDLH, the level of protection required may be downgraded, but as with choking agents, only if the respirator to be used is known to protect against the particular blood agent. SM 5-18 UNIT 5: PROTECTION It is common belief that hydrogen cyanide is a skin-absorbable toxin. Although this is partially true, the following provides some information that is not commonly known about hydrogen cyanide (HCN). The most common form of cyanide is a solid, but HCN is found as a liquid, and as a gas. When in liquid form the concentration varies between 2 percent and 99.5 percent. One of the problems with chemical information found with research texts is that typically it is for one form of the material, but the hazards that one form may present may not apply to another form. The exposure limits are as follows STEL--4.7 ppm, threshold limit value (TLV)--10 ppm, LCLo-200 ppm/5 mins. The skin-absorbable toxicity of cyanides generally comes from contact with the solid material. Through studies it is known that normal healthy humans have 0.5 mg/kg in their bodies most of the time. After an exposure, the halflife of cyanide in a human is 20 minutes to 1 hour. Studies also have shown that concentrations above 90 ppm can be fatal, and that 45 ppm can cause diverse symptoms. One experiment used a 12 kg dog and a 70 kg man who were exposed to HCN in a chamber containing 500 to 635 ppm. The dog began to show symptoms at 50 seconds, was unconscious in 75 seconds, and developed respiratory arrest at 93 seconds. At 91 seconds the man had no symptoms and left the chamber. After 3 to 8 minutes he developed minor symptoms, which continued for a period of time. The dog was left and assumed to be dead, but 12 hours later was found to have recovered with no lasting effects. All of the previous exposures included both skin and respiratory routes. Some testing was done specifically on skin absorption alone. Dogs were tested, and an exposure of 15,200 ppm HCN for 47 minutes was fatal to the dogs. However an exposure of 5,000 ppm for 180 minutes showed no effects. In one incident several workers were exposed while wearing gas masks and no other protective clothing. They were in the atmosphere for 8 to 10 minutes. They were incapacitated for 2 to 3 days but recovered without any apparent effects. They were exposed to 20,000 ppm, which is a considerable amount. This incapacitation suggests that HCN is toxic by skin absorption, but requires a high level to cause any effects. It can be derived then that your level of PPE should consider the amount present in the atmosphere. If persons without PPE of any type are alive (even symptomatic) within a given environment, then a responder with respiratory protection and some other type of PPE (chemical suit--A, B, C, or TOG) has more than adequate protection to make a rescue. (Information obtained from NIOSH report 77-108, Biological effects of exposure to hydrogen cyanide.) SM 5-19 EMERGENCY RESPONSE TO TERRORISM: TACTICAL CONSIDERATIONS: HAZARDOUS MATERIALS SM 5-20 UNIT 5: PROTECTION Activity 5.1 Selecting PPE Purpose To provide insight into selecting appropriate PPE based on the chemical and physical properties of the agent involved. Directions 1. Work in small groups. 2. Read through the five MSDS's and the permeation chart provided in this exercise. 3. For each agent, indicate the level of chemical and respiratory protection that would be best suited for an incident involving that agent. 4. The amount of chemical is less than 1 gallon and the haz mat team is going in to sample the material. The weather is cloudy, 68F (20ºc), wind is 3 mph, and humidity is 70 percent. 5. Document the factors you used when making your decisions on level of protection. 6. A spokesperson from your group will read your answers and list of factors considered to the rest of the class. Be prepared to explain your reasoning. SM 5-21 EMERGENCY RESPONSE TO TERRORISM: TACTICAL CONSIDERATIONS: HAZARDOUS MATERIALS Agent A Description Vapor pressure Volatility Vapor density Specific gravity Melting point Ionization potential Boiling point Route of entry Effects Symptoms Reacts Decomposes Solubility Protection Detection devices Treatment Decontamination PEL TLV STEL LD50 LC50 IDLH LCt50 ICt50 SM 5-22 Greenish-yellow gas 5,000 mm/Hg 20,000,000 mg/m3 2.5 -67F (-55ºC) -33F (-36.ºC) Respiratory Immediate Strong eye, throat, and mucous membrane irritation, coughing, choking, tightness in chest With many substances, is a corrosive and an oxidizer Slightly in water Respiratory pH, colorimetric tubes, Agent A electrochemical gas sensor Remove from environment, fresh air Fresh air, flush with water if contaminated with liquid 0.5 ppm 0.5 ppm 1 ppm 10 ppm ~6500 ppm 1 min. ~600 ppm UNIT 5: PROTECTION Agent B Description Vapor pressure Volatility Vapor density Specific gravity Melting point Ionization potential Boiling point Route of entry Effects Symptoms Reacts Decomposes Solubility Protection Detection devices Treatment Decontamination PEL TLV STEL LD50 LC50 IDLH LCt50 ICt50 Brown liquid 2.9 mm/Hg @ 25C (77ºF) 20,000 mg/m3 @ 25C 5 1.07 -70F (56.7ºC) <10.6 eV 310F (154ºC) Respiratory, skin absorption Immediate Runny nose, tightness of the chest, gastrointestinal symptoms will appear first Stable Quickly in acids and alkalis, slowly in water; decomposes in 3 hours at 302F (150ºC) Slightly in water, more in organic solvents Respiratory and skin Basic life support, decontamination Fresh air, flush with water if contaminated with liquid 0.000017 ppm 9.3 mg/kg 0.03 ppm 12 ppm 8 ppm SM 5-23 EMERGENCY RESPONSE TO TERRORISM: TACTICAL CONSIDERATIONS: HAZARDOUS MATERIALS Agent C Description Vapor pressure Volatility Vapor density Specific gravity Melting point Ionization potential Boiling point Route of entry Effects Symptoms Reacts Decomposes Solubility Protection Detection devices Treatment Decontamination PEL TLV STEL LD50 LC50 IDLH LCt50 ICt50 SM 5-24 Colorless liquid 760 mm/Hg @ 100C (212ºC) 22,933 mg/m3 1 1 34F (1.1º) 12.4 eV 100C or 212F Ingestion, respiratory Delayed Convulsions, tremors, loss of muscle control With many substances, can release hydrogen during a reaction Into hydrogen In water Respiratory Remove from environment, fresh air Fresh air, flush with water if contaminated with liquid 25 g/Kg UNIT 5: PROTECTION Agent D Description Vapor pressure Volatility Vapor density Specific gravity Melting point Ionization potential Boiling point Route of entry Effects Symptoms Reacts Decomposes Solubility Protection Detection devices Treatment Decontamination PEL TLV STEL LD50 LC50 IDLH LCt50 ICt50 Colorless to brown liquid 0.11 mm/Hg @ 25C (77ºC) 600 mg/m3 5.5 57F (13.9ºC) <10.6 eV 423F (217.2ºC) Skin Delayed Burning of skin 0.0005 ppm 0.0005 ppm 231 ppm 23 ppm SM 5-25 EMERGENCY RESPONSE TO TERRORISM: TACTICAL CONSIDERATIONS: HAZARDOUS MATERIALS Agent E Description Vapor pressure Volatility Vapor density Specific gravity Melting point Ionization potential Boiling point Route of entry Effects Symptoms Reacts Decomposes Solubility Protection Detection devices Treatment Decontamination PEL TLV STEL LD50 LC50 IDLH LCt50 ICt50 SM 5-26 Colorless gas 1,180 mm/Hg 6,000,000 mg/m3 3 -288F (-177.8ºC) 46F (7.8ºC) Respiratory Immediate for high levels, delayed for lower levels Coughing, choking, tightness in chest With water Slightly in water Slightly in water, soluble in benzene and acetic acid Respiratory Remove from environment, fresh air Fresh air, flush with water if contaminated with liquid 0.1 ppm 0.1 ppm 2 ppm 800 ppm 400 ppm UNIT 5: PROTECTION Permeation Chart Agent Suit 1 Suit 2 Suit 3 Boot 1 Boot 2 Glove 1 Glove 2 A B C D E >50 >20 >480 >480 NT >480 255 >480 NT 29 >480 <20 >480 >183 >480 35 >480 >480 NT 60 >480 120 100 300 300 >8 >480 1 >480 250 >480 240 >480 5 NT SM 5-27 EMERGENCY RESPONSE TO TERRORISM: TACTICAL CONSIDERATIONS: HAZARDOUS MATERIALS SM 5-28 UNIT 5: PROTECTION Selecting PPE Worksheet Agent A B C D E Level-Suit Suit Boot Gloves Chemical General Factors Considered Specific Factors Considered Agent A: Agent B: Agent C: Agent D: Agent E: Notes SM 5-29 EMERGENCY RESPONSE TO TERRORISM: TACTICAL CONSIDERATIONS: HAZARDOUS MATERIALS SM 5-30