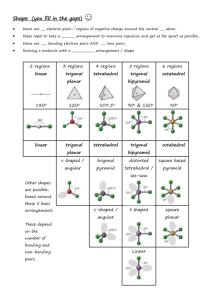
The 23 Most Asked Questions About Intermolecular Forces and
advertisement

The 23 Most Asked Questions About Intermolecular Forces and Energies. 1. What is the difference between kinetic and potential energy? Potential energy is the energy a particle has by virtue of its position; kinetic energy is the energy is has by virtue of its velocity, i.e., motion. 2. What “common and easily measurable” property can characterize the kinetic energy of the particles in a piece of matter? What exactly does it tell you? Temperature can characterize the kinetic energy of the particles in a piece of matter, although it will only tell you the average kinetic energy. Consequently, some particles will have more or less kinetic energy than is described by the temperature. 3. What is the relationship between force and potential energy? Why are we so casual about saying “London forces” and then drawing a curve for a potential energy? The mathematical expression for the force is given by the negative spatial (position) derivative of the expression for the potential energy. Physically, this means that the force tells you how fast the potential energy is changing as a function of position and nothing about the absolute size of the potential energy. Since we can derive one from the other to within a constant, however, they are very similar ways to describe the same physical phenomenon and are often used interchangeably. 4. How does the physical property “work” relate forces to kinetic and potential energy? Work is defined as the integral of the force with respect to the position. If the force being integrated is the negative derivative of the potential energy (like F=–kx for Hooke’s law), then (not surprisingly) the resulting to work is equal to the negative change in PE. On the other hand, if we use the force as F=ma, the work is equal to the change in kinetic energy. If the change in KE is not equal to the negative change in PE, then there are nonconservative forces involved in this process, like friction. 5. Since dipoles have both positive and negative ends, why are dipole-dipole forces attractive? When dipoles can change their orientation (either by fluctuating or rotating) they will tend to align themselves so that the overall potential energy of the system will be minimized. This means that permanent rotating dipoles will arrange themselves so that the ends of opposite charges are closer on the average. On the other hand, fluctuating dipoles will actually be created in a favorable orientation as a response to a nearby dipole. (As an aside, exactly why the system would tend to lower overall potential energy is not clear from anything we have said thus far. Essentially, when the system happens to move to a lower potential energy state, the change in energy becomes kinetic energy which escapes in the form of heat. We’ll discuss this further when we do thermodynamics.) 6. What are dispersion or London forces? Describe how they arise. Atoms and molecules are tiny highly charged positive centers surrounding by a “cloud” of negative electrons. As the electrons move around the cloud changes shape and “instantaneous” dipoles are created. These dipoles will then induce dipoles in nearby atoms and molecules with the net effect of allowing for a favorable dipole-dipole interaction. We call these forces between fluctuating dipoles dispersion or London forces. 7. What do we mean by Van der Waals forces? Van der Waals forces are the general term we use to describe all dipole-dipole forces, i.e., those with the 1/r6 potential energy, whether they are generated by induced or rotating dipoles. 8. Why do the forces generated by permanent dipoles and those generated by induced dipoles both have a –1/r6 potential energy expression? The reason why both cases have this form is because they both consist of dipoles that can change their orientation and on the average interact favorably. The actual math that shows why we get the 1/r6 form is beyond the scope of this course. (However, it is important to note that the 1/r6 form is the result of averaging the orientation of the dipoles. If the dipoles have a fixed orientation, as they do in solids, their interaction can be much stronger.) 9. Draw a typical intermolecular potential energy curve. What are the physical sources of the repulsive and attractive parts? What is the one case where two particles would have a very different intermolecular interaction? 2 1.5 1 0.5 0 0.9 1.1 1.3 1.5 1.7 1.9 2.1 -0.5 (I generated this curve in Excel just because I needed something electronic to put in these answers. In case you’re curious, the equation I plotted is: V (r ) 1 1 6 12 r r The short range repulsions are generated by the interaction of the electron clouds when the particles get close. The long range attractions have several possible sources such as van der Waals forces, electrostatic attraction, covalent bond formation, hydrogen bonding, or some combination of these. The one exception to this kind of interaction is like-charged ions which have a long range electrostatic repulsion. 10. What is the physical reason why the typical repulsive forces are so short ranged? These forces are caused by the overlap of the electron clouds and the particles have to actually “touch” for this to happen. 11. What is the hydrogen bond and under what circumstances does it occur? The H-Bond is a special interaction that can occur between a partially positive (acidic) hydrogen of one molecule and the lone pair on an electronegative element of another molecule. The interaction is about 20 kJ/mole, between van der Waals (2 kJ/mole) and ion-ion (250 kJ) interaction. It is shorter ranged than van der Waals forces and also has a strong angle dependence, forcing the molecules involved to achieve a careful alignment for the interaction to take effect. 12. Rank the common intermolecular interactions (Van der Waals, electrostatic, covalent bond, hydrogen bond) in terms of strength and range. Decreasing Strength: Covalent, electrostatic, H-bond, VdW. Decreasing Range: Electrostatic, VdW, H-bond, covalent (the last two are close). 13. What are cohesive forces? Describe the nature of cohesive forces in molecular liquids (polar and non-polar) and ionic solids. How do these forces explain their relative melting and boiling points? Cohesive forces are the general name for the forces that hold molecules together in the condensed phases, i.e., solids and liquids. In ionic solids, these forces are predominantly the ion-ion electrostatic interactions, whereas in molecular liquids they are complete VdW and maybe H-bonding (polar liquids) or simply dispersion forces (non-polar liquids). The stronger the attractions the higher the melting and boiling points. Thus, we would expect ionic compounds to have higher melting and boiling points than molecular ones, and hydrogen bonded ones to be in between. (Although covalent bonds are the strongest interactions of all, they are usually intramolecular and not intermolecular forces. There are exceptions where covalent bonds do serve as cohesive forces, most notably metals which are effectively held together with a special kind of weak, extended covalent bonds. Metals melt at fairly high temperatures but still lower than most ceramics. Diamond is an example of a substance held together with full strength covalent bonds. Diamond doesn’t really melt but does sublimate at 5100K!) 14. Explain the statement “you need attractive forces to condense and repulsive forces to freeze”. As mentioned above, the attractive forces actually cause the molecules in a gas to collapse to a much denser solid or liquid state. The difference between a solid and a liquid, on the other hand, is that the molecules in a solid are fixed in space whereas those in a liquid can move around. It is the short range repulsions that hold the molecules in place in the solid state, because these are the interactions that represents what happens when two particles come in contact. (To hold something in place, even in common speech, means to put it in contact with enough other objects such that it no longer has any place to move.) 15. What is the basic “law of mixing”? Explain what it means. “Like dissolves like”. This means that polar or ionic solutes will tend to dissolve in polar solvents and non-polar solutes will tend to dissolve in non-polar solvents. Any attempt to mix one into the other to any significant extent will disrupt the dominate forces of each component. (We use the word “component” when we don’t want to designate either substance in a mixture as solvent or solute.) For example, a non-polar hydrocarbon molecule in water would get between water molecules and prevent hydrogen bonding from occurring. Since these interactions lower the energy of the liquid, it is better for the “mixture” to exist as two separate layers. (Again, notice that we see the tendency of a system to try to minimize its energy.) 16. Explain the “charge in a sphere” interpretation of the equation describing the Coulomb force. The Coulomb force is described by the equation: 1 q1q2 F (r ) 4 r 2 where q1 and q2 are the charges of the interacting particles, r is the distance between them, and is the permittivity, which is essentially the strength of the Coulomb force in a particular medium. The charge in sphere interpretation is to think of q1 as “seeing” q2 smeared out over a sphere of radius r. Since the area of this sphere gets larger with increasing r, the charge per unit area, q2/4r2, will get increasingly smaller. Hence, the interaction between q1 and this smeared out charge will get smaller as 1/r2 as the separation gets larger. 17. Explain why NaCl can dissolve in H2O. How is this different from the dissolution of a molecular solute like ethanol (C2H5OH)? In the ionic case, the solute will break up into the individual ions, e.g., Na+ and Cl- . Water can overcome the strong electrostatic interaction between the ions by replacing the presence of the other ion with water molecules supplying “countercharge” from the appropriate end of its molecular dipole. With a molecular solute, the molecule is intact and will try to simply “replace” a water molecule in the overall structure of the solvent. This is especially effective for water if the solute can undergo hydrogen bonding. 18. How can water put NaCl in the liquid state almost 800 degrees below its melting point? Use the concepts of kinetic and potential energy in your explanation. In order to melt NaCl, you must supply kinetic energy, i.e., raise the matter to a sufficiently high temperature, to overcome the binding energy between the ions. This energy is given by the depth of the potential energy well. In the dissolution process, the ions are separated not by pulling them apart with kinetic energy but by supplying alternate sources of potential energy to replace the binding energy.