ESCS Final Exam Fall Study Guide Name: Period:______ Date
advertisement

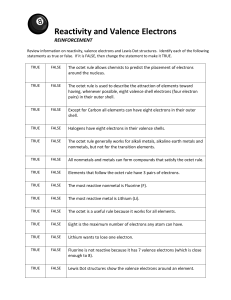
ESCS Final Exam Fall Study Guide Name: _________________________________________________ Period:___________ Date: ____________ 1. What do we call a pure substance made of one type of atom that can NOT be broken down? A mixture contains the elements sulfur and iron. Sulfur Iron Not Soluble in water Not soluble in water Solid Yellow powder smells like rotten eggs Boils at 444.674 º C Solid Dark metal that is magnetic Boils at 2750 º C. 2. Using the physical properties in the chart, how would it be easiest to separate the mixture of sulfur and iron? 3. What is the difference between a heterogeneous and homogeneous mixture? 4. What do we call the ability of one substance to dissolve in another substance? 5. What does the Kinetic Theory state? 6. What must have happened if a chemical change has occurred? 7. What factors affect the pressure of an enclosed gas? 8. What can be learned about an atom from the following: a. Its atomic number: b. Its mass number: 9. What electrical charges do protons, neutrons, and electrons have and where can each be found? 10. What is an isotope? 11. What causes an electron to jump to a new energy level? 12. Why do atoms lose or gain electrons? 13. How many valence electrons are in each group (family) of the periodic table? 14. Be able to define and give examples of the following: a. Alkali Metals e. Transition Metals b. Alkaline Earth Metals f. Metals c. Halogens g. Nonmetals d. Noble Gases h. Metalloids 15. List some of the properties and characteristics of metals: 16. List some of the properties and characteristics of nonmetals: 17. What kinds of elements share some of the properties of both metals and nonmetals? 18. What are ions, cations and anions? 19. How many valence electrons do the most reactive elements tend to have? 20. What is the difference between an ionic bond and a covalent bond? 21. Which kinds of elements typically combine in such a way that electrons are transferred? 22. Which kinds of elements typically combine in such a way that electrons are shared? 23. Which kinds of elements typically form cations? Why and how? 24. Which kinds of elements typically form anions? Why and how? 25. When electrons are transferred, which elements do the “giving” and which do the “taking”? 26. Know what happens when an element becomes an ion. a. When potassium becomes an ion, it: b. When nitrogen becomes an ion, it: 27. Be able to determine the number of atoms that must be combined with another atom to form a stable compound. For example: a. Write the formula for how lithium atoms combine with oxygen atoms to make lithium oxide. b. Write the formula for how beryllium atoms and chlorine atoms combine to make beryllium chloride. 28. Know how to draw Bohr models and Lewis dot models for elements 1 – 20 on the periodic table. a. Li e. P b. Mg f. O c. B g. Cl d. C h. Ne 1 / 1A H 2.1 Li 1.0 Na 0.9 K 0.8 Rb 0.8 Cs 0.8 Electronegativity 2 / 2A Be 1.6 Mg 1.3 Ca 1.3 Groups 3 - 12 13 / 3A B 2.0 Al 1.6 14 / 4A C 2.5 Si 1.9 15 / 5A N 3.0 P 2.2 16 / 6A O 3.5 S 2.5 17 / 7A F 4.0 Cl 3.0 Br 2.8 I 2.7 18 / 8A He Ne Ar Kr Xe - Keeping in mind, if the electronegativity difference is:: -less than or equal to 0.5 the bond is nonpolar covalent -between 0.5 and 2.0 the bond is considered polar covalent -greater than or equal to 2.0 the bond is ionic 29. Predict what kind of bond will form between the following: a. Lithium and fluorine b. Carbon and Oxygen c. Fluorine and Fluorine d. Carbon and Hydrogen 30. Which elements typically form a positive charge when bonding? 31. Which elements are least likely to undergo bonding? 32. How do the following atoms achieve a completely filled outer shell of electrons when they form ionic bonds? What valence or oxidation number (Charge) does each of these have? a. Lithium c. Boron b. Beryllium d. Carbon 33. e. Nitrogen g. Fluorine f. Oxygen h. Neon Write the valence or oxidation number (Charge) for each of the following elements or ions, and then use the criss cross method to write the chemical formula for combining them into compounds: a. Sodium and oxygen b. Beryllium and chlorine c. Aluminum and sulfur d. Magnesium and Chlorine 34. Use Lewis dot structures to decide how many electrons are shared by the atoms in each of the following diatomic molecules; identify each of these as a single, double, or triple covalent bond: a. F2 c. O2 b. Cl2 d. N2 35. What does a chemical formula represent? 36. What is polyatomic ion? 37. What is the formula for: a. Ammonium Phosphate c. Magnesium Chloride b. Lithium Carbonate d. Diphosphorus Triselenide ESCS Final Exam Fall Study Guide KEY 1. What do we call a pure substance made of one type of atom that can NOT be broken down? An element A mixture contains the elements sulfur and iron. Sulfur Iron Not Soluble in water Not soluble in water Solid Yellow powder smells like rotten eggs Boils at 444.674 º C Solid Dark metal that is magnetic Boils at 2750 º C. 2. Using the physical properties in the chart, how would it be easiest to separate the mixture of sulfur and iron? Use a magnet to separate out the iron 3. What is the difference between a heterogeneous and homogeneous mixture? Homogeneous is uniform throughout; heterogeneous is “lumpy” or inconsistent 4. What do we call the ability of one substance to dissolve in another substance? Solubility 5. What does the Kinetic Theory state? All particles of matter in constant motion. 6. What must have happened if a chemical change has occurred? New substance has been formed. 7. What factors affect the pressure of an enclosed gas? Temperature, Volume, & # of Particles 8. What can be learned about an atom from the following: a. Its atomic number: how many protons, and in neutral state how many electrons b. Its mass number: sum of protons plus neutrons; weighted average of isotope mass 9. What electrical charges do protons, neutrons, and electrons have and where can each be found? Proton is positive; neutron is neutral; electron is negative; proton & neutron are in nucleus; electrons are outside the nucleus 10. What is an isotope? Same number of protons, different number of neutrons 11. What causes an electron to jump to a new energy level? 12. Why do atoms lose or gain electrons? Atom gains or loses energy To become more stable (octet rule) 13. How many valence electrons are in each group (family) of the periodic table? Group 1/1A has 1; group 13/3A has 3, etc.) 14. Be able to define and give examples of the following: Tied to group # (e.g. a. Alkali Metals Group 1 c. Halogens Group 17 b. Alkaline Earth Metals Group 2 d. Noble Gases Group 18 15. e. Transition Metals Groups 3-12 f. Metals everything left & below staircase (except H) g. Nonmetals everything right & List some of the properties and characteristics of metals: above staircase h. Metalloids on and immediately below staircase conductors, malleable, ductile, solids at room temp 16. List some of the properties and characteristics of nonmetals: insulators, brittle 17. What kinds of elements share some of the properties of both metals and nonmetals? metalloids 18. What are ions, cations and anions? Ions: any electrical charge; cations: positive; anions: negative 19. How many valence electrons do the most reactive elements tend to have? One or seven 20. What is the difference between an ionic bond and a covalent bond? Ionic transfers electrons; covalent shares 21. Which kinds of elements typically combine in such a way that electrons are transferred? Metals to nonmetals 22. Which kinds of elements typically combine in such a way that electrons are shared? Nonmetals with nonmetals 23. 24. 25. 26. Which kinds of elements typically form cations? Why and how? Metals; octet rule; give up electrons to get to zero in valence shell Which kinds of elements typically form anions? Why and how? Nonmetals; octet rule; borrow electrons to get full valence shell (eight) When electrons are transferred, which elements do the “giving” and which do the “taking”? Metals (with few valence electrons) give (lend) and nonmetals (with many electrons) take (borrow). Know what happens when an element becomes an ion. a. When potassium becomes an ion, it: gives up an electron becomes a cation (with 1+ charge) b. When nitrogen becomes an ion, it: borrows 3 electrons and becomes an anion (with 3- charge) 27. Be able to determine the number of atoms that must be combined with another atom to form a stable compound. For example: a. Write the formula for how lithium atoms combine with oxygen atoms to make lithium oxide. Li gives up e- and becomes Li+ cation; O takes on 2 e- and becomes O2- anion; criss cross for Li2O b. Write the formula for how beryllium atoms and chlorine atoms combine to make beryllium chloride. Be gives up 2 e- and becomes Be2+ cation; Cl takes on 1 e- and becomes Cl- anion; criss cross for BeCl2 28. Know how to draw Bohr models and Lewis dot models for elements 1 – 20 on the periodic table. a. Li Bohr: ‘Li’ center; 1st shell 2 e-; 2nd shell 1 eLewis: ‘Li’ center and 1 dot b. Mg Bohr: ‘Mg’ center; 1st shell 2 e-; 2nd shell 8 e-; 3rd shell 2 e-; Lewis: ‘Mg’ center and 2 dots c. B Bohr: ‘B’ center; 1st shell 2 e-; 2nd shell 3 eLewis: ‘B’ center and 3 dots d. C Bohr: ‘C’ center; 1st shell 2 e-; 2nd shell 4 eLewis: ‘C’ center and 4 dots 29. e. P Bohr: ‘P’ center; 1st shell 2 e-; 2nd shell 8 e-; 3rd shell 5 e-; Lewis: ‘P’ center and 5 dots f. O Bohr: ‘O’ center; 1st shell 2 e-; 2nd shell 6 eLewis: ‘O’ center and 6 dots g. Cl Bohr: ‘Cl’ center; 1st shell 2 e-; 2nd shell 8 e-; 3rd shell 7 e-; Lewis: ‘Cl’ center and 7 dots h. Ne Bohr: ‘Ne’ center; 1st shell 2 e-; 2nd shell 8 eLewis: ‘Ne’ center and 8 dots Predict what kind of bond will form between the following: e. Lithium and fluorine (4.0 – 1.0 = 3.0) Ionic f. Carbon and Oxygen (3.5 – 2.5 = 1.0) Polar Covalent g. Fluorine and Fluorine (4.0 – 4.0 = 0.0) Nonpolar Covalent h. Carbon and Hydrogen (2.5 – 2.1 = 0.4) Nonpolar Covalent 30. Which elements typically form a positive charge when bonding? Metals 31. Which elements are least likely to undergo bonding? Noble Gases (already have full shell) 32. How do the following atoms achieve a completely filled outer shell of electrons when they form ionic bonds? What valence or oxidation number does each of these have? a. Lithium Loses 1 e-; valence 1+ b. Beryllium Loses 2 e-; valence 2+ c. Boron Loses 3 e-; valence 3+ d. Carbon Loses 4 e-; valence 4+ e. Nitrogen Gains 3 e-; valence 3f. Oxygen Gains 2 e-; valence 2g. Fluorine Gains 1 e-; valence 1h. Neon Full shell – no change 33. Write the valence or oxidation number for each of the following elements or ions, and then use the criss cross method to write the chemical formula for combining them into compounds: a. Sodium and oxygen Na+ and O2- Na2O b. Beryllium and chlorine Be2+ and Cl- BeCl2 c. Aluminum and Sulfur Al3+ and S2- Al2S3 d. Magnesium and Chlorine Mg2+ and Cl- MgCl2 34. Use Lewis dot structures to decide how many electrons are shared by the atoms in each of the following diatomic molecules; identify each of these as a single, double, or triple covalent bond: a. F2 c. O2 F – F each F has 7 dots & 1 shared pair Single covalent bond b. Cl2 Cl – Cl each Cl has 7 dots & 1 shared pair Single covalent bond O = O each O has 6 dots & 2 shared pairs Double covalent bond d. N2 N = N each N has 5 dots & 3 shared pairs Triple covalent bond 35. What does a chemical formula represent? Number or ratio of atoms in compound 36. What is polyatomic ion? Covalently bonded unit that carries a charge 37. What is the formula for: a. Ammonium Phosphate b. Lithium Carbonate (NH4)3(PO4) Li2(CO3) c. Magnesium Chloride MgCl2 d. Diphosphorus Triselenide P2Se3