1 INSTRUCTION For Medical Application of the Preparation of
advertisement

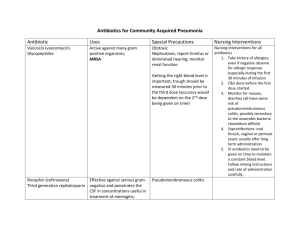
1 INSTRUCTION For Medical Application of the Preparation of KANAMYCIN Trade name of the preparation: Kanamycin International nonproprietary name: kanamycin Dosage form: powder for compounding solution for intramuscular and intracavitary administration Composition for one vial: kanamycin disulphate (in equivalent of kanamycin) – 0.5 g, 1.0 g. Description: powder of white color Pharmacotherapeutic group: antibiotic-amino glycosides ATC code [J01GB04] Pharmacodynamics It is an antibiotic with a wide spectrum of a group of amino glycosides. In low concentration it renders bacteriostatic action (by means of damage of protein synthesis in microbial cells), in high concentration it renders bactericidal action (it damages a cytoplasmic membrane of the microbial cell). It penetrates in a microbial cell, interlinks with the specific proteins-receptors on 30S ribosome subunit. It disturbs formation of complex of transport and messenger RNA (30S ribosome subunit) and stops synthetic process of proteins. It is effective in regard to majority of gram-positive and gram-negative microorganisms as well as acidresistant bacteria: Mycobacterium tuberculosis (including resistant to streptomycin, paraaminosalicylic acid), isoniazid and other antituberculous preparations, except viomycin), Escherichia coli, Shigella spp., Salmonella spp., Proteus spp., Enterobacter spp., Klebsiella pneumoniae, Neisseria gonorrhoeae et meningitidis, Staphylococcus spp. Strains of those microorganisms resistant to tetracycline, erythromycin, chloramphenicol, benzylpenicillin, streptomycin and others, in most cases preserve sensibility to kanamycin. It has no effect on Pseudomonas aeruginosa, Streptococcus spp., Bacteroides spp. and other anaerobic bacteria, yeast fungus, viruses and protozoans. Pharmacokinetics Maximum concentration of kanamycin disulphate in blood plasma in intramuscular injection is achieved in 0.5-1.5 hour and amounts 22 µg/ml in dosage preparation of 7.5 milligram/kilogramme. It penetrates with a pleural space, lymphatic, synovial and peritoneal liquid, blood serum, bronchial secretion and bile. Time of achievement of maximum concentration in bile is 6 hours. High concentrations are found in urina; low concentrations are found in bile, human milk, aqueous humor, bronchial secretion, expectoration and cerebrospinal fluid. It easily penetrates in all tissues of the organism, where it accumulates intracellular; high concentrations are registered in organs with good blood supply: lungs, liver, myocardium, lien, and especially in kidneys, where it accumulates in cortex, more lower concentrations are found in muscles, fatty tissues and bones. In norm the kanamycin does not work way through a hematoencephalic barrier, nevertheless in in- 2 flammation of brain-tunic the concentration of the preparation in cerebrospinal fluid reaches 30-60 % of such in blood plasm. In newborn infants higher concentrations are formed in cerebrospinal fluid, comparing that of the adults; it passes through placenta (it is found in fetal blood and amniotic fluid). The volume of distribution in adults is 0.26 l/kg, in children is 0.2-0.4 l/kg, in newborn infants at the age of less than 1 week and body weight less than 1.5 l/kg the volume of distribution is up to 0.68 l/kg, at the age of less than1week and body weight more than 1.5 kg the volume of distribution is up 0.58 l/kg, in patients with mucoviscidosis the value is 0.3-0.39 l/kg. It does not metabolize. The half-life period in adults is 2-4 hours, in newborn infants is 5-8 hours, in children of more older age is 2.5-4 hours. The final half-life period is more than 100 hours (it releases out of intracellular depot). It is egested by kidneys by the way of glomerular filtration predominantly in an unaltered form (70-95 % is found in urina in 24 hours). The half-life period in adults in kidney malfunction varies depending on degree of the malfunction to 100 hours, in patients with mucoviscidosis -1-2 hours, in patients with burns and hyperthermia the half-life period can be shorter in comparison with the mean value on account of the elevated clearance. It is egested in haemodialysis (50 % within 4-6 hours), peritoneal dialysis is less effective (25 % within 48-72 hours). Indications to application Infectious diseases induced by microorganisms sensitive to kanamycin. Severe pyogenic-septical diseases: sepsis, meningitis, peritonitis, bacterial endocarditis. Infectious-inflammatory diseases of respiratory organs: pneumonia, empyema, lung abscess. Infections of kidneys and urinary tracts: pyelonephritis, cystitis, urethritis. Suppurative complications in post-operation period, infected burns and other disease, induce predominantly by gram-negative microorganisms (Escherichia coll, Enterobacter aerogenes, Serratia, Salmonella spp., Klebsiella pneumoniae, Proteus spp., Shigella spp.), resistant to other antibiotics or associations of grampositive and gram-negative causative agents. Tuberculosis of the lungs and tuberculous damages of other organs (as apart of complex therapy), induced by Koch's bacillus resistant to antituberculous preparation of the I and II lines and other antituberculous preparations, except florimycin. Contra indications Supersensitiveness to kanamycin (including other amino glycosides in anamnesis), severe chronic kidney disease with azotemia and uremia, neuritis of the VIII pair of cerebral nerves, pregnancy. With care Myasthenia, parkinsonism, botulism (amino glycosides can induce damage of neuromuscular transmission, resulting in further weakening of skeletal muscles), renal insufficiency, elderly age, prematurely born children, neonatal period (up to 1 month), period breast feeding. Application in pregnancy and in the period of breast feeding Application in pregnancy is contraindicated. In the period of breast feeding the preparation should be applied with care, when potential usefulness for the 3 mother exceeds potential risk for the infant. Mode of administration and dosages Intramuscular. Before administration the content of the vial 0.5 gram or 1.0 gram dissolve in 2 or 4 milliliters of water for injection respectively or 0.25-0.5 % procaine solution (Novocaine). For treatment of infections of nontuberculous ethiology a single dose of intramuscular injection for adults is 0.5 gram, a daily dose is 1-1.5 gram (0.5 grams every 8-12 hours). The maximum daily dose is 2 grams (1 gram in 12 hours). The duration of the treatment is usually 5-7 days depending on severity and characteristic property of the process run. Children of the first month of life and prematurely born can be injected the preparation only for intravital indications in a daily dose of 15 mg/kg. Children at the age of 1 month to 1 year are administered with an average daily dose of 0.1 gram, from 1 to 5 years – 0.3 gram, above 5 years – 0.3-0.5 gram. The maximum daily dose is 15 mg/kg. The daily dosage should be divided into 2-3 injections. The duration of the treatment is usually 5-7 days depending on severity and characteristic property the process run. In case of renal insufficiency the schedule of administrations should be corrected by way of dosage decrease or interval increase between the administrations. For calculation of the intervals between injections taking into account damage level of the kidney function the following formula can be recommended: interval between administrations in hours is equal to the creatinine content in blood plasma (mg/100ml) x 9. Dosage calculation: An initial dose is calculated taking into account the body weight (dose in mg = body weight x 7) Initial dose Subsequent doses =----------------------------------------------------------------------------------Content of creatinine in the blood serum (mg/100 ml) in frequency of administration 2-3 times per day On the days of haemodialysis after its carrying out additionally administer a single dose of the preparation. In the time of treatment of tuberculosis (as part of complex therapy) the preparation should be administered to adults 1 time daily in dose of 1 gram or 0.5 gram twice per day, the children should be administered 15mg/kg/day but not more than 0.5-0.75 gram 6 days a week with a break on the seventh day. The number of cycles and total duration of the treatment is determined by the stage and characteristic properties of the disease run and totals 1 month. In order to avoid overdosage of the preparation it is recommended to periodically control concentration of the antibiotic in the patient’s blood. Intracavitary. In peritonitis: in an abdominal cavity for lavement administer 10-50 ml 0.25 % water solution; intra- 4 peritoneal by 500 mg (in the form of 2.5 % water solution); in carrying out peritoneal dialysis dissolve 1-2 gram in 500 milliliters of dialyzing liquid. In empyema of pleural membrane: administer 10-50 milliliters of 0.25 % water solution in pleural space for lavement. In abscess cavity for lavement administer 10-50 milliliters of 0.25 % water solution. The daily dose in intracavitary administration should not exceed a daily dose for intramuscular injection. The duration of intracavitary administration is determined by characteristic properties of the disease run. Side effects On the part of gastrointestinal tract: nausea, vomit, diarrhea, compromised liver function (increment in activity of "hepatic" transaminases, hyperbilirubinemia). On the part of blood-making organ: anaemia, leukopenia, granulocytopenia, thrombocytopenia. On the part of nervous system: headache, drowsiness, weakness, neurotoxic action (clonus, benumbed sensation, tingling, paresthesia, epileptic attacks), possible violation of neuromuscular transmission (respiratory standstill). On the part of sense organs: ototoxicity (ring or sensing loading in ear, decrease of sense of hearing down to inconvertible deafness), toxic effect on vestibular apparatus (incoordination movement, paraequilibrium, nausea, vomit). On the part of urinary system: nephrotoxicity - malfunction of kidneys (increase or decrease of urinary frequency, thirst, cylindruria, microscopic hematuria albuminuria). Hypersensitivity reactions: exanthema, itch, dermahemia, fever, Quincke's disease. Overdosage Symptoms: toxic reactions (hearing loss, ataxia, paraequilibrium, urinary discomfort, thirst, anorexia, nausea, vomit, ring or sensing of loading in ears, breathing trouble). Treatment: for reversal of blockade of neuromuscular transmission and its consequences - haemodialysis or peritoneal dialysis, dissociable inhibitors of choline esterase, calcium salts, artificial pulmonary ventilation, other symptomatic and maintenance therapy. Interaction between other medicinal preparations. It is pharmaceutically incompatible with streptomycin, gentamicin, penicillins, heparin, cephalosporin, capreomicin, amphotericin A, erythromycin, aminohydantoin, viomycin. Nalidixic acid, polymyxin B, cisplatin and vancomycin increase risk of development of oto- and nephrotoxicity. Beta-lactam antibiotics (cephalosporin, penicillins) in patients with severe chronic kidney disease decrease effect of amino glycosides. Diuretics (especially furosemide), cephalosporins, penicillins, sulfanilamides and nonsteroidal antiinflammatory preparations, competing for active secretion in canaliculi of nephron, block elimination of aminoglycoside, raise their concentration in blood serum, strengthen nephro- and neurotoxicity. 5 Parenteral administration of indometacin increases risk of development of poisonous action of kanamycin (increase of the half-life period and decrease of clearance). It decreases effect of antimyasthenic medicinal preparations. It strengthens miorelaxing actions of nondepolarizing muscle relaxants, general anesthetics and polymyxins. Methoxyflurane, polymyxin for parenteral administration and other medicinal preparation, block neuromuscular transmission (halogenated hydrocarbons in the capacity of preparations for inhalational anesthesia, narcotic analgesics, transfusion of ample quantity of blood with citric preservatives) increase risk of emergence of nephrotoxic actions and respiratory standstill (as a result of strengthening of neuromuscular block). Cyclopropane increases risk of development of apnoea in intraperitoneal administration of kanamycin. Special instructions In the period of treatment it is necessary to control function of kidneys, a cochlear nerve and a vestibular apparatus no less than once per week. Probability of development of renal toxicity is higher in patients with parafunction of kidneys, as well as in application of high doses or application for a long period of time (that category of the patients may need daily inspection of the kidneys function). In case of the unsatisfactory audiometric test results the dosage of the preparation should be decreased or the treatment be ceased. Amino glycosides penetrate with human milk in small numbers (because they are poorly soaked from the gastrointestinal tract, no complications associated with them have been reported in sucking children). The patients with infectious- inflammatory disease of urinary tracts are recommended to take increased amount of liquid. In case of failure of positive clinical dynamics one should remember about possibility of development of resistant microorganisms. In such cases it is necessary to abolish treatment and start carrying out corresponding medical maintenance. Impact on control of transport vehicles and mechanisms. It is necessary to exercise caution when running operations requiring elevated concentrations of attention and quickness of psychomotor action. Product form The powder for compounding a solution for intramuscular and intracavitary administration 0.5 gram, 1 gram. 0.5gram and 1 gram of active matter are contained in each of 10 ml capacity vials. Each carton has 50 vials with equal number of the application data sheets for supply to inpatient hospitals. Storage conditions In a dry place at temperature not exceeding 25°C. Keep out of reach of children. 6 Period of validity 4 years. Do not use after expiry date indicated on the package. Pharmacy purchasing terms Prescription medicine. Holder of the Registration Certificate/Manufacturer/Organization accepting reclamations Open Joint Stock "Kurgan Joint Stock Company of Medical Preparations and Articles "Sintez" (Sintez JSC) No. 7, Prospect Konstitutsii, city of Kurgan, Russian Federation, 640008 Tel/fax (3522) 48-16-89 e-mail: real@kurgansintez.ru Web site: http://www.kurgansintez.ru