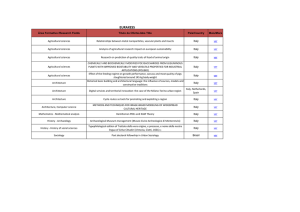
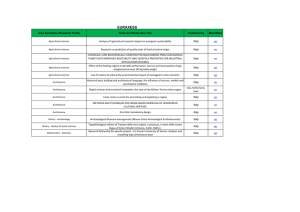
contoh 2 (sample 2)
advertisement

BORANG PERMOHONAN KELULUSAN ETIKA PELAJAR PASCA IJAZAH (M.Med, M.Sc & Ph.D) PUSAT PENGAJIAN SAINS PERUBATAN (PPSP) ETHICAL APPROVAL APPLICATION FORM for POSTGRADUATE STUDENT (M.Med, M.Sc & Ph.D) PPSP Nota:(Notes):1. Borang permohonan terdiri daripada 8 mukasurat. (Application form consists of 8 pages). 2. Contoh Carta Aliran Projek dan Carta Gantt sila rujuk mukasurat 9 - 11. (Example of ‘Project Flow Chart ’and ‘Gantt Chart’ refer page 9 - 11). 3. Senarai kumpulan FOR rujuk mukasurat 12 - 14 dan kumpulan SEO dimukasurat 15 untuk rujukan sahaja [tidak perlu dicetak]. (List of FOR group refer page 12 – 14 and SEO group refer page 15).For reference only [no need to print]. 4. Permohonan hendaklah dimajukan ke Pejabat Bhg. Siswazah, PPSP untuk diperakukan oleh Dekan dan Timbalan Dekan (Pengajian Siswazah & Latihan Iktisas), PPSP. Please send the form to the Postgraduate Office, School of Medical Sciences for endorsement by the Dean and Deputy Dean (Postgraduate and Professional Training), School of Medical Sciences. 5. Selepas mendapat perakuan Dekan dan Timbalan Dekan (Pengajian Siswazah & Latihan Ikhtisas), PPSP, serahkan 3 salinan borang ini (termasuk salinan asal) ke Pejabat Pelantar Penyelidikan Sains Klinikal, USM untuk tindakan lanjut. (Following the approval from the Dean and Deputy Dean (Postgraduate and Professional Training), School of Medical Sciences, please make 3 copies (including original copy) and send to Clinical Science Research Platform Office, USM for further action). FOR POSTGRADUATE OFFICE’S USE ONLY Stamp Date received (From PG Student): Received by: Signature: Stamp Date received (From Dean’s Office): Received by: Signature: Stamp Date received (Research Platform Office): Received by: Signature: JEPeM/EthicalForm01/Ver.3 - 2010 Updated: 15/12/2010 1 CHECKLIST FOR ETHICAL APPROVAL (To be submitted together with the Application Form) ; Postgraduate Office, PPSP Note: 1. 2. 3. PI or Co-researchers has to defend their research proposal. Student dissertation is required in the research proposal. Supervisor or Co-supervisor must be present during student’s presentation of research proposal. No. Project Title 1. Name of Postgraduate Student 2. Specialty / Area 3. Name of Main Supervisor 4. Name of Co-supervisors / Co-researchers 5. Location of Research 6. Duration of this research Please Answer the Following Questions 7. Has similar study been done before? (If yes, when?) 8. Research Problem / Rationale / Justification of the study given. 9. Objectives 10. Hypothesis 11. Study design 12. Sample size calculation 13. Detailed methodology 14. References 15. Flow Chart of research 16. Gantt Chart of research activities 17. Expected results 18. Benefit of the study 19. Budget 20. Pages are numbered 21. Informed consent / subject information M.Med Dissertation / M.Sc / Ph.D i) ii) iii) Yes / No / Not Applicable a. Ethical issues (Animal / Human) (if yes, please specify either Animal or Human or Both) 22. b. Approval from Health Ministry or other Ministries required? (If yes, please specify) 23. Discussion at Department: (Attach a copy of the Attendance Sheet signed by Head of Dept) Verification and Comment by Student’s Supervisor JEPeM/EthicalForm01/Ver.3 - 2010 Updated: 15/12/2010 2 ATTENDANCE SHEET (To be filled up at department level) Date: __________________ Nama Name No. 1. Tandatangan Signature Ketua Jabatan: Head Department: 2. 3. 4. 5. 6. 7. 8. 9. 10. Endorsed by: …………………………. (Head of Department) Stamp: JEPeM/EthicalForm01/Ver.3 - 2010 Updated: 15/12/2010 3 BORANG ETIKA - 01 JAWATANKUASA ETIKA PENYELIDIKAN (MANUSIA) - JEPeM RESEARCH ETHICS COMMITTEE (HUMA N) PERMOHONAN KELULUSAN ETIKA (ETHICAL APPROVAL APPLICATION) (Sila sampaikan 3 salinan kertas kerja ini (termasuk salinan asal) kepada Pejabat Pelantar Penyelidikan Sains Klinikal, Kampus Kesihatan USM melalui Dekan / Pengarah / Ketua Jabatan di Pusat Pengajian masing-masing) (Please submit 3 copies (including original sopy) of your research proposal to Clinical Research Platform Office, USM Health Campus through your respected Deans / Head Department) Tajuk penyelidikan yang dicadangkan: Title of proposed research A. Nama: Name No. Kad Pengenalan: Identity Card No. Pusat Tanggungjawab [PTJ]: School / Department / Unit No. Matrik: Matric Number No. Telefon Pejabat: Office Telephone No. Alamat E-mel: E-mail Address Jawatan: Position Tahun Pengajian: Year Tarikh mula berkhidmat dengan Universiti ini: Date of first Appointment to the University Jika kontrak, nyatakan tarikh tamat: If on contract, state expiry date Pelantar Penyelidikan dipilih ( √ ): Selected Research Platform Clinical Research Engineering & Technology Fundamental Sciences Biomedical & Health Sciences Social Transformation Life Sciences Information & Comm. Technology B (i). Kumpulan FOR (Field of Research Group) *(Sila rujuk Panduan / Please refer to " Malaysian (R&D) Classification System”) Kumpulan SEO (Socio-Economic Objective Group) *(Sila rujuk Panduan / Please refer to "Malaysian (R&D) Classification System") Kod FOR: Kod SEO: Tempat penyelidikan dijalankan: Location of research Tempoh projek (tidak melebihi 24 bulan): Duration of project (not more than 24 months) *Terdapat di Pusat Pengajian masing-masing atau layari http://www.research.kk.usm.my/borang/MRDCS_5th_Edition.pdf *Available at your School or go to http://www.research.kk.usm.my/borang/MRDCS_5th_Edition.pdf JEPeM/EthicalForm01/Ver.3 - 2010 Updated: 15/12/2010 4 B (ii). Ringkasan Penyelidikan Summary of Research Proposal * [Perlu meliputi latar belakang dan penyataan masalah, tujuan penyelidikan, kaedah penyelidikan (sertakan carta alir dan carta gantt), hasil yang dijangkakan dan senaraikan penerbitan yang berkaitan (jika ada).] *[Must include the background and problem statement, objectives, research methodology (please include flow chart), expected outcome and list of related publications] (a) Tujuan (Objectives) (b) Latarbelakang (Background) (c) Kaedah (Methodology) - Sila sertakan carta alir dan carta Gantt (Please provide a flowchart and Gantt chart) (d) Hasil (Expected Outcome) (e) Penerbitan Berkaitan (Related publication) * Jika terdapat maklumat tambahan, sila sertakan. * Please attach additional information if necessary B (iii). B (iv). Kepentingan dan faedah Penyelidikan The importance and the benefits of the research Alat - alat dan bahan yang terdapat di Universiti untuk kegunaan penyelidikan ini. Equipment and materials available in the University to be used for this research. JEPeM/EthicalForm01/Ver.3 - 2010 Updated: 15/12/2010 5 C (i). Jika penyelidikan ini dijalankan bersama dengan penyelidik lain, nyatakan peranan mereka: If this research is conducted together with other researchers, please state their role: Nama dan No. Kad Pengenalan Name and Identity Card No. D. Pusat Pengajian/Jabatan/Unit School/Department/Unit Tandatangan Signature Sumbangan/ Peranan dalam kajian Role in this research Pengakuan Declaration Saya mengaku bahawa semua maklumat yang diberi adalah benar dan cadangan penyelidikan ini tidak dipohon untuk geran lain. I hereby declare that all information provided are accurate and that this proposal has not been submitted to any other grant. Tarikh: ……………………. Date JEPeM/EthicalForm01/Ver.3 - 2010 Updated: 15/12/2010 Tandatangan Pemohon: ……………………. Applicant’s Signature 6 E. Komen Ketua Jabatan: Head Department’s Comments i. Relevance - Have similar studies been done before? Yes/No. If Yes, where and how will this research add value? - How will this research contribute to the schools/university/society’s aspirations? ii. Approach - Is this research practical and deliverable? - Does the proposed methodology have sufficient scientific and research merit in its knowledge domain? - Does the proposal methodology have any ethical issue. Please elaborate. .................................................. Tandatangan Ketua Jabatan Head Department’s Signature ............................... Tarikh Date Cop Rasmi: Official Stamp JEPeM/EthicalForm01/Ver.3 - 2010 Updated: 15/12/2010 7 F. Komen Timbalan Dekan (Pengajian Siswazah & Latihan Ikhtisas): Deputy Dean’s (Postgraduate & Professional Training) Comments ....................................................................... Tandatangan Timbalan Dekan (Pengajian Siswazah & Latihan Ikhtisas) Deputy Dean’s (Postgraduate & Professional Training) Signature ............................... Tarikh Date Cop Rasmi: Official Stamp G. Komen Dekan: Dean’s Comments ............................................ Tandatangan Dekan Dean’s Signature ............................... Tarikh Date Cop Rasmi: Official Stamp JEPeM/EthicalForm01/Ver.3 - 2010 Updated: 15/12/2010 8 CONTOH 1 (SAMPLE 1) Flow Chart for Experimental Design (A) <TITLE OF THE STUDY> 5,000 Blood Donors (HUSM & GHKB) Routine Screening Anti-HCV 4,900 HCV-ve 100 HCV +ve Random Selection of 50 Samples Random Selection of 50 Samples RT-PCR Test For HGV-RNA PCR-ELISA for Confirmation Agroser Gel Electrophoresis Statistical (B) <TITLE OF THE STUDY> Haemodialysis Subjects Multiply Transf. Subjects Intravenous Drug Users Chronic & Acute Viral Hepatitis n=20 n=30 n=40 n=10 100 Serum Samples ELISA HGV-RNA JEPeM/EthicalForm01/Ver.3 - 2010 Updated: 15/12/2010 9 CONTOH 2 (SAMPLE 2) (C) <TITLE OF THE STUDY> This is a cross-sectional study in which a total of 360 household where selected from mukims in Pasir Puteh and Tanah Merah by multistage random sampling. 45 samples will be collected from each type of water supply system (gravity feed, overhead tank, direct connector and others). The household will be interview by the researcher and research assistant/health inspector/public health assistant regarding the water quality and water samples will be taken 2 times from each house. KELANTAN Multistage 6 DISTRICT (with all 4 types of water supplies available) Random sampling Pasir Puteh Tanah Merah Mukims GFS n=45 OHT n=45 DC n=45 Mukims OTHER n=45 GFS n=45 OHT n=45 DC n=45 OTHER n=45 This water samples will be taken to KMAM (Drinking Water Quality Control) unit laboratory in District Health Office for analysis of physical, chemical and microbiological contamination. The results will be compared with the standard value of drinking water from Ministry of Health to get the percentage of contamination violation Data entry, analysis, and interpretation by using the EPI INFO 6.04 and SPSS Software (version 9.0) Report and paper preparation for presentation JEPeM/EthicalForm01/Ver.3 - 2010 Updated: 15/12/2010 10 CONTOH 3 (SAMPLE 3) Gantt Chart of Research Activities: <Title of The Study> PROJECT ACTIVITIES Research Activities 2009 S O N 2010 D J F M A M J J 2011 A S O N D J F M A M Patient / Subjects Recruitment Data Collection Data Analysis / Interpretation Presentation & Submission of Reports Report Writing Project Completed Submission of Research Papers Milestone of Research Activities: 1. 2. 3. 4. 5. End of March 2010 : Completion of Phase 1 Data Collection Sept. 2010 : Completion of Phase 2 Data Collection End of Sept. 2010 : Data Analysis April 2011 : Preparation of Research Presentation Oct. 2011 : Report submission JEPeM/EthicalForm01/Ver.3 - 2010 Updated: 15/12/2010 11 J J 2012 A S O N D J F M A M J LAMPIRAN A (ATTACHMENT A) LIST FIELD OF RESEARCH (FOR) Category F1100000 Medical and Health Sciences F1100100 Immunology F1100101 F1100102 F1100103 F1100104 F1100105 F1100107 F1100108 F1100199 Allergy Cellular immunology Humeral immunology Immunochemistry Immunogenetics Transplantation immunology Tumor immunology Other immunology F1100200 Medical biochemistry and clinical chemistry F1100201 Carbohydrates F1100204 Lipids F1100205 Nucleic acids Peptides F1100206 Proteins F1100299 Other medical biochemistry and clinical chemistry F1100300 Medical microbiology F1100301 Bacteriology F1100302 Mycology F1100303 Parasitology F1100304 Virology F1100399 Other medical microbiology F1100400 Pharmacology F1100401 F1100402 F1100405 F1100407 F1100499 Clinical pharmacology Medical pharmacology Pharmaceutical field Pharmaceutical research Pharmaceutical technology Pharmacy Radiopharmacology Radiotoxicology Therapeutics Traditional medicine and natural product Other pharmacology Physiology F1100501 F1100502 F1100503 F1100504 F1100506 F1100507 F1100599 Cell physiology Comparative physiology Exercise/physical fitness Human biophysics Stress physiology Systems physiology Other physiology Neurosciences F1100601 F1100602 F1100603 F1100604 F1100605 F1100606 F1100607 F1100608 F1100599 Autonomic nervous system Cell receptors Cellular nervous system Central nervous system Mechanism of tissue damage Molecular characterization Peripheral nervous system Sensory systems Other neurosciences F1100404 F1100500 F1100600 JEPeM/EthicalForm01/Ver.3 - 2010 Updated: 15/12/2010 12 F1100700 Clinical sciences F1100701 Allergies F1100702 Anesthesia Arthritis F1100703 Carcinogenesis F1100704 Cardiology Child health Dentistry F1100706 Dermatology F1100708 Endocrinology Fetal development Fetal medicine F1100710 Gastroenterology F1100711 Geriatrics F1100712 Gynecology Hearing therapy F1100713 Hematology F1100714 Infectious/communicable diseases F1100715 Intensive care F1100716 Medical genetics F1100717 Neonatology F1100718 Nephrology F1100719 Neurology F1100720 Neuromuscular diseases F1100721 Nuclear medicine F1100722 Obstetrics F1100723 Occupational therapy F1100724 Oncology F1100725 Ophthalmology Optometry Oral surgery Organ imaging F1100726 Orthopedics F1100727 Otolaryngology F1100729 Pathology F1100728 Paediatrics Pharmaceuticals Pharmacy F1100730 Physical therapy F1100731 Psychiatry Psychology F1100732 Radiology F1100733 Radiotherapy F1100734 Rehabilitation F1100735 Reproduction F1100736 Respiratory diseases F1100737 Rheumatology Speech therapy F1100738 Surgery Therapy F1100740 Urology F1100741 Venereology F1100799 Other clinical sciences F1100800 Public health, environmental & occupational health & safety research F1100801 Air quality F1100802 Drinking water quality F1100803 Environmental protection & health impact F1100804 Epidemiology Food quality F1100805 Health & safety (occupational) F1100806 Health information systems Infant Immunisation F1100807 Injuries F1100808 Mental health F1100810 Preventive medicine F1100811 Primary health care F1100812 Radiation health & safety Solid waste management F1100813 Urban health F1100815 Vector borne diseases F1100816 Waste water collection & treatment F1100899 Other public health, etc JEPeM/EthicalForm01/Ver.3 - 2010 Updated: 15/12/2010 13 F1100900 Nutrition (public health research) F1100901 Clinical epidemiological Laboratory Toxicology F1100999 Other nutrition (public health research) F1101000 Health services research (including bioethics) F1101001 Bioethics (human) Education (health) Health & community services F1101002 Health care administration F1101004 Health education & promotion F1101005 Health systems management Human bioethics Mortality and morbidity studies Nursing F1101099 Other health services F1101100 Health cares systems, industries and technologies F1101101 Biomechanics F1101104 Delivery systems Emergency medicine F1101108 Health legislation F1101111 Health rehabilitation service F1101114 Human resource development F1101115 Innovative technology F1101116 Medical biotechnology Medical technology Quality control of medical diagnostic instruments Quality of life F1101119 Other health care system, industries and technologies F1101199 F1101200 Pharmaceutical industry F1101201 Botanical pesticides F1101205 Photochemical F1101207 Purification technology F1101208 Screening technology F1101209 Synthesis technology F1101299 Other pharmaceutical industry F1109900 Other medical and health services not elsewhere classified JEPeM/EthicalForm01/Ver.3 - 2010 Updated: 15/12/2010 14 SOCIO-ECONOMIC OBJECTIVE (SEO) Category S30100 Health S3010100 Clinical (organs, diseases and conditions) S3010101 Blood disorders S3010102 Bone & Joint disorders S3010103 Cancer S3010104 Cardiovascular diseases Chronic hepatitis Digestive system S3010107 Endocrine diseases S3010109 Hearing, vision & speech S3010111 Immune system & allergy S3010112 Infectious diseases S3010113 Inherited diseases S3010114 Kidney diseases S3010116 Neurological disorders Orthodontics conditions Reproductive medicine S3010122 Respiratory diseases S3010123 Sexuality transmitted disease and HIV infection Skin & related conditions S3010124 Surgical methods Vaccines Vector-borne diseases S3010125 Other clinical health S3010199 S3010200 Public health S3010203 S3010206 S3010211 S3010212 S3010213 S3010214 S3010215 S3010216 S3010217 S3010218 S3010220 S3010221 S3010222 S3010224 S3010226 S3010227 S3010299 S3010300 S30199 JEPeM/EthicalForm01/Ver.3 - 2010 Dental health Disease distribution & transmission Environmental health Food safety (public health) Health & women Health & behavior Health & children Health & indigenous people Health & social structure Health & the ageing Health (public) indicators Malignancies Maternal & child health Mental health Occupational health Preventive medicine Substance abuse (health) Urban health Other public health Health and support services S3010304 Diagnostic methods/kids S3010311 Health education & promotion S3010312 Health policy economic outcomes S3010313 Health policy evaluation S3010316 Nursing care S3010318 Occupation therapy S3010320 Palliative care S3010322 Physiotherapy Speech therapy S3010399 Other health and support services Other health not elsewhere classified Updated: 15/12/2010 15