Chapter 2
advertisement

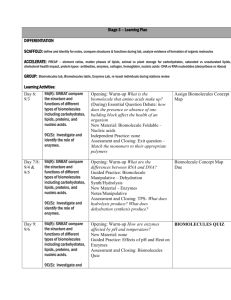
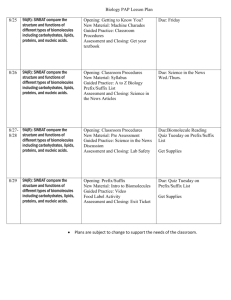
______________________’s notes Biology Chapter 2:The Chemistry of Life Section 2-1: Basic Chemistry What is the basic unit of matter? __________________________ What are the three subatomic particles? 1. _____________________ (p+) – (_____) charge; weighs 1 amu 2. _____________________ (n0) – __________ charge (0); 1 amu 3. _____________________ (e-) – (_____) charge; 1/1840 amu Section 2-1: Basic Chemistry What subatomic particles are in the nucleus? A. __________ & _________ B. Overall charge of the nucleus is ________________ (+) What subatomic particles are outside of the nucleus? A. _______________________ B. Electrons are constantly moving within orbits known as _________________. What is the charge of an atom? ______________ because the number of protons equals the number of electrons. -1- Section 2-1: Basic Chemistry What is an element? Pure substance that is made of only _______ kind of atom. Periodic Table Basics: 1. _______________________________________ = # of p+; number of e2. _______________________________________ = number of p+ & n0 Section 2-1: Basic Chemistry What is an isotope? A. An atom of an element with an ___________________________________________. 1. Isotopes are identified by their _______________________ 2. Isotopes have the same chemical properties because they have the normal number of e- These isotopes of carbon are named or identified by their different MASS numbers. All isotopes of carbon have the same atomic number. Section 2-1: Basic Chemistry What are radioactive isotopes? Isotopes that have a _________________________________________________. What are radioactive isotopes used for? 1. _________________________________________________________ 2. _________________________________________________________ -2- 3. __________________________________________________________ 4. Used as a label or “______________________” to follow molecules as they move through a cell. Section 2-1: Basic Chemistry What is a compound? 1. Substance formed by the chemical combination of ____________________________ _________________________________________________________________ 2. The physical & chemical properties of a compound are different than the elements that it is made from. Section 2-1: Basic Chemistry What are valence electrons? 1. e- found in the ______________________________________ of an atom 2. They are used to __________________________________________________. Section 2-1: Basic Chemistry What is an ionic bond? 1. Chemical bonds in which ___________________________________ from one atom to another. 2. This makes one atom (________) and the other atom (________). 3. These charged atoms are called ______________, and they are attracted to each other. 4. Ionic compounds _________________________ in water. -3- Section 2-1: Basic Chemistry What is a covalent bond? 1. Chemical bond in which ____________________________________ between atoms. 2. e- are shared in ____________. 1 pair = ________________________; 2 pair = ________________________; 3 pair = ________________________ 3. Compounds that have covalent bonds are called __________________________. 4. _________________________________. Section 2-1: Basic Chemistry What are van der Waals forces? 1. The __________________________ between molecules when they are close together. 2. The feet of ______________________ use these forces to hang on to smooth surfaces. Section 2-2: Chemistry of Water What is cool about H2O? 1. It is the _________________________________________________ in living things. 2. Most chemical reactions occur in a ________________________________________. 3. It __________________________________ when it freezes. Section 2-2: Chemistry of Water What is polarity? 1. The _____________________________________________________ in a molecule. -4- 2. The _____________________ atom of H2O is an ________________________. 3. The _____________________ of the molecule has a partial (_____________) charge & the _____________________ has a partial (__________) charge. 4. All of the special properties of H2O are related to its _______________________. Section 2-2: Chemistry of Water What is a hydrogen bond? 1. It is the attraction between the partial (+) of one water molecule to the partial (-) of another. 2. H2O can form ___________________________ of these at once. 3. _______________________________ represent a H+ bond. Section 2-2: Chemistry of Water What is cohesion? 1. The attraction of H2O molecules to ________________________________________. 2. ____________________________ form between the molecules. 3. ____________________________ is caused by this. -5- Section 2-2: Chemistry of Water What is adhesion? 1. The attraction of H2O molecules __________________________________________. 2. _______________________________ are formed between the H2O and the substance. 3. _______________________________________ is caused by this. Section 2-2: Chemistry of Water What is a mixture? Two or more substances mixed together ________________________________. What is a solution? 1. A mixture in which something is dissolved in H2O. ___________________________ = does the dissolving 2. ___________________________ = gets dissolved H2O can dissolve both ________________ and ___________ compounds. Section 2-2: Chemistry of Water What is a suspension? 1. A mixture in H2O in which the substances ___________________________. 2. Anything that says “_______________________.” 3. _________________________ is a suspension. -6- Section 2-2: Chemistry of Water Dissociation of H2O: H2O can break down to form __________________. What is an acid? 1. Any substance that forms _______________________ in solution 2. _____________________ on the pH scale 3. Strong acids have a pH of ___________________. Section 2-2: Chemistry of Water What is a base? 1. Any substance that forms _____________________ in solution. 2. _________________ on the pH scale 3. Also called ______________________________. 4. Strong bases have a pH of _________________________. What is a buffer? 1. Substances that _______________________________________________________. 2. Many are ____________________________________________________________. 3. Many _______________________________ act as buffers. Section 2-2: Chemistry of Water What is the pH scale? A measurement of the ______________________________________________ in a solution. It is based on powers of 10. Base: _____________ Neutral = ____________ Acid: ______________ -7- pH scale Section 2-2: Chemistry of Water What is a Buffer? The pH of the fluids within most cells in the human body must generally be kept between ______________ in order to maintain _________. If the pH is lower or higher, it will affect the chemical reactions that take place within the cells. One of the ways that organisms control pH is through dissolved compounds called _____________, which are weak acids or bases that can react with strong acids or bases to _____________sharp, sudden changes in pH. Section 2-3: Biomolecules What is organic chemistry? The study of ______________________________. What are carbon’s most important traits? 1. ___________________________________________. This allows carbon to make ____________________________________ at once. 2. _____________________________________________________________________. This allows it to form chains, rings, chains of rings, or a combination of all. -8- Section 2-3 What are the major elements of life? ____________________, ______________________, ___________________, ____________________, _______________________, ___________________ Section 2-3: Biomolecules What is a hydrocarbon? The basic carbon compound. It is made of only ______________ and ______________. Benzene Methane Section 2-3: Biomolecules What are functional groups? Compounds attached to carbon chains to give them special properties. Hydroxyl (-OH) Amino (-NH2 or Carboxyl (-COOH or ) ) ____________ Alcohols ____________ Amino Acids ____________ Amino Acids & Fatty Acids Section 2-3: Biomolecules What is a macromolecule? 1. “Big” molecule; ________________________________________________ 2. _____________________________________________ are macromolecules 3. Made of individual subunits What is the subunit of a macromolecule? -9- 1. ____________________________. ______________ = one; ________ = part 2. ____________________ = two parts; 2 monomers joined together 3. ______________________ = 3 or more parts; 3 or more monomers joined together; macromolecules are polymers Section 2-3: Biomolecules What is polymerization? 1. Chemical process in which ___________________________________ to form dimers & polymers. 2. A.K.A. _____________________________________ because ________ is produced. Dehydration = ____________________________; synthesis = __________________. Take away H2O to make something. Sometimes called ________________________. 3. It is an ____________________________ process because it takes energy & builds new molecules. 4. ___________________________________ groups are used for this process. Section 2-3: Biomolecules What is hydrolysis? 1. Chemical process in which the ____________________ between the monomers in a polymer are ____________________. 2. A _____________________ molecule is used. 3. Hydro = _____________; lysis = __________; using water to break something. 4. It is a _____________________ reaction because energy is released and molecules are broken. - 10 - Section 2-3: Biomolecules What are the four biomolecules? 1. ________________________________ 2. ________________________________ 3. ________________________________ 4. ________________________________ Section 2-3: Biomolecules What is a carbohydrate? 1. Molecules that contain C, H, and O in a ____________ ratio (glucose = C6H12O6) 2. Carbo = __________________; hydrate = ______________ What is the monomer of a carbohydrate? 1. ____________________________ 2. Mono = _____________________; saccharide = _____________________________ 3. All sugars end with ____________________ (glucose, fructose, lactose, sucrose, etc.). Section 2-3: Biomolecules What are disaccharides? 1. __________________________________________ joined together. 2. This is done by ____________________________. 3. ________________________ (table sugar) is an example. - 11 - What is a polysaccharide? 1. _____________________ monosaccharides joined together through polymerization. 2. _____________________ & ____________________ are examples. Section 2-3: Biomolecules What are the functions of carbohydrates? 1. ______________________________________ for cells, especially ______________. 2. ___________________________. Plants use __________; animals use ___________. 3. ___________________________. Plant cell walls are made of _________________, the exoskeletons of insects & crabs are made of ___________________. Section 2-3: Biomolecules What are lipids? 1. Compounds that contain C, H, and O, but _______________________________ ratio. 2. They are ___________, ___________, ___________, & ___________. 3. None are soluble in H2O. 4. They have no real monomer. - 12 - 5. A ______________________________________ is the form of a typical fat or oil. Tri refers to 3 fatty acids; glyceride refers to glycerol. Section 2-3: Biomolecules What is a saturated fatty acid? 1. The hydrocarbon chain only has ____________________ between the carbons (C-C). 2. It has the maximum amount of ____________________________ (saturated w/H). 3. Tend to be _____________________at room temperature (fats). Section 2-3: Biomolecules What is an unsaturated fatty acid? 1. The hydrocarbon chain has _______________________ between two carbons (C=C). 2. It has __________________________ the maximum hydrogens. What is a polyunsaturated fat? 1. The hydrocarbon chain has _________________________. 2. They tend to be ________________________ at room temperature (oils). - 13 - Section 2-3: Biomolecules What are the functions of lipids? 1. _________________________________ – fats in animals 2_________________________________________________________ (phospholipids) 3. __________________________________ (hormones). Many steroids are hormones. 4. ____________________________________________________ (waxes). Section 2-3: Biomolecules What are proteins? 1. Arguably the most important biomolecule. 2. Proteins usually end with ___________ (insulin, hemoglobin, adrenalin, etc.) Section 2-3: Biomolecules What is the monomer of a protein? ____________________________ What are the parts of an amino acid? 1. __________________________________________ (center carbon atom) - 14 - 2. __________________________________________ (acid group)(-COOH) 3. __________________________________________ (-NH2) 4. __________________________________________ (H) 5. __________________________________________; this is a variable; there are 20 different R groups; 20 different amino acids Section 2-3: Biomolecules How are amino acids joined together? 1. By a ___________________________________ formed through polymerization. 2. The amino group of one amino acid is connected to the carboxyl group of another. Section 2-3: Biomolecules What is a dipeptide? ____________________________ joined together. - 15 - What is a polypeptide? _____________________________________ joined together. Most proteins are polypeptides made of at least 300 amino acids. What determines a protein’s function? ______________________________ Section 2-3: Biomolecules What are the levels of protein structure? 1. ____________________ (1o) – amino acid sequence; this determines the next 3 levels 2. ____________________ (2o) – the amino acid chains coils and folds 3. ____________________ (3o) – the 2o structure folds on itself 4. ____________________ (4o) – one or more polypeptide chains are added; a.k.a. globular protein; hemoglobin is an example. Section 2-3: Biomolecules What are the functions of proteins? 1. ______________________________________ – after carbs & lipids have been used. 2. __________________________ – microtubules & microfilaments give shape to cells. 3. _____________________________ – actin & myosin are used in muscle contraction. 4. ______________________________________________ – hemoglobin transports O2 5. ____________________________________ – many hormones are proteins (insulin) 6. ____________________________________ – antibodies are proteins 7. _________________________________ – these proteins regulate chemical reactions; this is the most important function of proteins. Section 2-3: Biomolecules What are nucleic acids? 1. Compounds that contains C, H, O, N, & P. 2. _______________________________________________________________ (DNA) 3. ________________________________________________________ (DNA & RNA) 4. _______________________________________________________________ (ATP) Section 2-3: Biomolecules - 16 - What is the monomer of a nucleic acid? ______________________________ What are the parts of a nucleotide? 1. _______________________________________ (either ribose or deoxyribose) 2. _______________________________________ 3. _______________________________________ (5 different kinds of these) Section 2-3: Biomolecules Basic Nucleotide Structure: Basic ATP structure: Section 2-4: Chemical Reactions & Enzymes What is a chemical reaction? 1. Process that changes one set of chemicals into another set of chemicals. 2. Always involves _____________________________________________________ & _____________________________________________________________. A+BC+D ___________________ (left of the arrow) ________________ (right of the arrow) The arrow means “_____________.” Section 2-4: Chemical Reactions & Enzymes What is activation energy (EA)? Energy required to start a chemical reaction. What is an anabolic reaction? 1. Absorbs ____________________; endergonic. 2. The energy is used to make _________________________. - 17 - 3. _______________________________________ is an anabolic reaction. 4. New molecules are built. Section 2-4: Chemical Reactions & Enzymes What is a catabolic reaction? 1. Releases ______________; exergonic 2. Energy is released because ______________________________________ are broken. 3. ____________________________ is a catabolic reaction. - 18 - Section 2-4: Chemical Reactions & Enzymes What is an enzyme? 1. Proteins that _____________________________________ by __________________. 2. Enzymes are _________________________; they are not used up during the reaction. 3. They are ______________________________; one enzyme per reaction. 4. Enzymes end with __________________. Section 2-4: Chemical Reactions & Enzymes Lock & Key Model of Enzyme Function: 1. Substrate = ________________ 2. _________________________ = place on the enzyme where the work occurs. It is substrate specific. 3. Enzyme-Substrate complex last for a very short time. - 19 - Section 2-4: Chemical Reactions & Enzymes How do you control enzymes? 1. ___________________________________ 2. _______________________________________; especially higher temperature 3. __________________________________________ – these block the active sites. Changes in __________________ & _____________________ changes the shape of the enzyme (__________________). - 20 -