ORGANIC REACTION TYPES:
advertisement
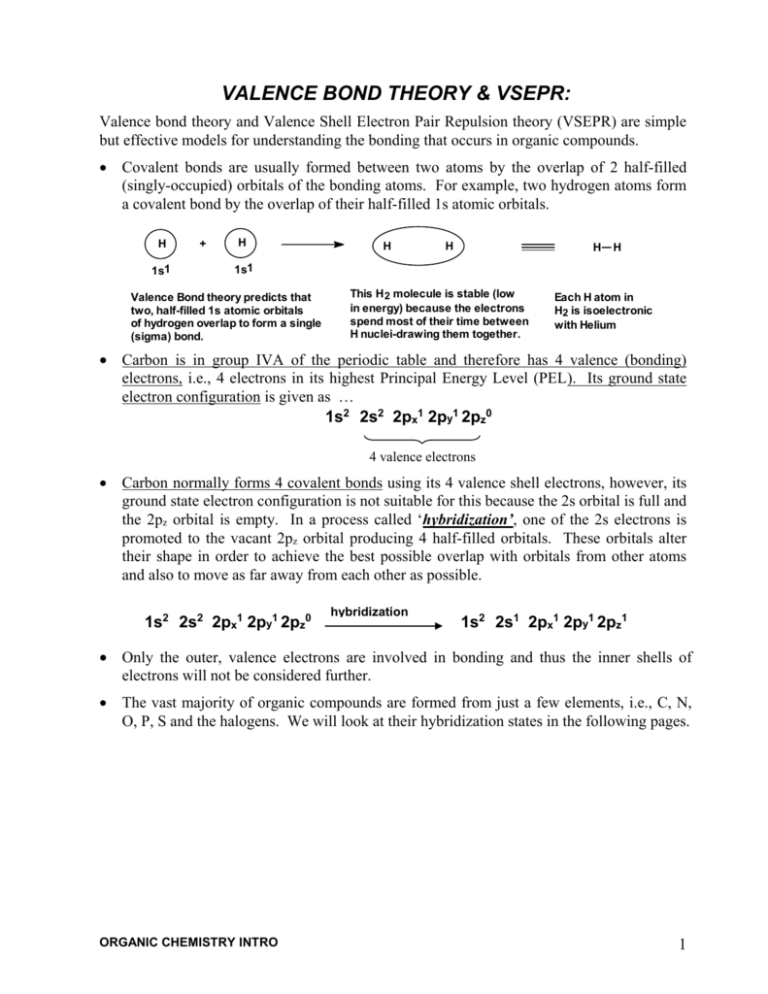
VALENCE BOND THEORY & VSEPR: Valence bond theory and Valence Shell Electron Pair Repulsion theory (VSEPR) are simple but effective models for understanding the bonding that occurs in organic compounds. Covalent bonds are usually formed between two atoms by the overlap of 2 half-filled (singly-occupied) orbitals of the bonding atoms. For example, two hydrogen atoms form a covalent bond by the overlap of their half-filled 1s atomic orbitals. H 1s1 + H H H H H 1s1 Valence Bond theory predicts that two, half-filled 1s atomic orbitals of hydrogen overlap to form a single (sigma) bond. This H 2 molecule is stable (low in energy) because the electrons spend most of their time between H nuclei-drawing them together. Each H atom in H2 is isoelectronic with Helium Carbon is in group IVA of the periodic table and therefore has 4 valence (bonding) electrons, i.e., 4 electrons in its highest Principal Energy Level (PEL). Its ground state electron configuration is given as … 1s2 2s2 2px1 2py1 2pz0 4 valence electrons Carbon normally forms 4 covalent bonds using its 4 valence shell electrons, however, its ground state electron configuration is not suitable for this because the 2s orbital is full and the 2pz orbital is empty. In a process called ‘hybridization’, one of the 2s electrons is promoted to the vacant 2pz orbital producing 4 half-filled orbitals. These orbitals alter their shape in order to achieve the best possible overlap with orbitals from other atoms and also to move as far away from each other as possible. 1s2 2s2 2px1 2py1 2pz0 hybridization 1s2 2s1 2px1 2py1 2pz1 Only the outer, valence electrons are involved in bonding and thus the inner shells of electrons will not be considered further. The vast majority of organic compounds are formed from just a few elements, i.e., C, N, O, P, S and the halogens. We will look at their hybridization states in the following pages. ORGANIC CHEMISTRY INTRO 1 BONDING IN CARBON COMPOUNDS 6 C orbital shape Group 4A 4 valence e’s. Covalence = 4 2py1 2px1 2s2 ground state valence electron configuration A change (hybridization) occurs to the orbital shape and electronic configuration to facilitate bonding. The 3 hybridizations occurring in cabon are shown below. 2pz0 sp 2 hybridized (triangular planar) sp hybridized (linear) Carbon forms a double bond Carbon forms 1 triple or 2 double bonds sp 3 hybridized (tetrahedral) Carbon forms 4 single bonds The arrangement of the 4 atomic orbitals and the electronic configuration in the carbon atom are not suitable to form 4 bonds. 180º 90º 109.5º or C 120º C C C orbital shape (sp2 orbitals x 3) + (p x 1) hybridized orbitals (sp orbitals x 2) + (p x 2) (sp3 orbitals x 4) electron configuration (3 s bonds) + (1 p bond) (forms 4 s bonds) alkanes e.g., ethane (C 2H6) bonds C C H H H H H C C H H H C H C H H H H H ORGANIC CHEMISTRY INTRO H H C H H H H C C H H H H H H H H H C alkynes e.g., acetylene (C 2H2) H H H H (2 s bonds) + (2 p bonds) alkenes and arenes e.g., ethene and benzene (C 2H4) (C 6H6) H H C C H also CO2 O C O 2 BONDING IN NITROGEN COMPOUNDS Group 5A 7 The shape and orientation of the 4 atomic orbitals in the nitrogen atom are not suitable for forming 3 (or 4) bonds. N orbital shape 5 valence e’s. Covalence = 3 2s2 ground state valence electron configuration 2py1 2px1 A change (hybridization) occurs to the orbital shape to facilitate bonding. The 3 hybridizations occurring in nitrogen are shown below. 2pz1 sp 3 hybridized sp 2 hybridized sp hybridized Nitrogen forms 3 or 4 single bonds Nitrogen forms a double bond Nitrogen forms 1 triple bond ...... .. .. 90º or .:. N N 107º orbital shape hybridized orbitals 180º N 120º .. .... .. (sp 2 orbitals x 3) + (p x 1) (sp3 orbitals x 4) (sp orbitals x 2) + (p x 2) electron configuration bonds N .. 1 lone pair + 3 s bonds .... or H N N H H (1 lone pair) + (2 s bonds) + (1 p bond) (4 s bonds) Cl H ammonium chloride NH3 NH4 Cl + - nitriles e.g., ethanenitrile N CH3 C ..: N (1 lone pair) + (1 s bond) + (2 p bonds) .. N amines ammonia ORGANIC CHEMISTRY INTRO .. + H H H azo compounds e.g., (trans azobenzene) .:. N .... N :N C CH3 3 BONDING IN OXYGEN COMPOUNDS 8 The shape and orientation of the 4 atomic orbitals in the oxygen atom are not optimal for forming 2 (or 3) bonds. O orbital shape Group 6A 6 valence e’s. Covalence = 2 2s2 ground state valence electron configuration 2px2 2py1 A change (hybridization) occurs to the orbital shapes to facilitate bonding. The 2 hybridizations occurring in oxygen are shown below. 2pz1 sp 3 hybridized sp 2 hybridized Oxygen forms 2 single bonds Oxygen forms a double bond O .. .. hybridized orbitals .:. .... O .... .. orbital shape 90º 105º or .. .. O : 120º .. (sp 2 orbitals x 3) + (p x 1) (sp3 orbitals x 4) electron configuration bonds 2 lone pair + 2 s bonds or e.g., methanol (CH 3OH) CH3 .... O .... e.g., acetone (CH3CCH3) + hydronium ion (H3O ) H .. O H ORGANIC CHEMISTRY INTRO (2 lone pair) + (1 s bonds) + (1 p bond) O 1 lone pair + 3 s bonds H ..: O ..: C + H H3C : O: C H3C CH3 CH3 4 Note that a double bond is made of one s and one p bond. Note that a triple bond is made of one s and two p bonds. Halogens (groups VIIA elements) generally form only one s bond in organic compounds. They do not reshape their orbitals (hybridize) when they bond. The shape and orientation of the 4 atomic orbitals in the halogens are adequate for forming one single bond. 17 Cl orbital shape ground state 3s2 valence electron configuration 3px2 3py2 (3 lone pairs and 1 s bond) H Cl Hybridization does not occur when halogens form single bonds. 3pz1 C H H .. : Cl .. CH3 methyl chloride (chloromethane) Hydrogen, like the halogens, does not hybridize its 1s orbital when bonding. Silicon, like carbon, is a group 4A element with 4 valence electrons. As expected, silicon forms sp3 hybridized tetrahedral compounds with 4 substituents. Simple examples include silicon tetrabromide (SiBr4) and tetramethylsilane [(CH3)4Si]. Silicon forms a few compounds in which it has double bonds, e.g., H2Si=CH2. However, silicon's large size makes p-orbital overlap for p bonds less effective than in carbon compounds. Unlike 2nd period elements which cannot accommodate more than 8 electrons in their valence orbitals, Si, a 3rd period element can expand its valence shell to accommodate 10 electrons (sp3d hybridized – 5 substituents, e.g., SiF5-) and even 12 electrons (sp3d2 hybridized – 6 substituents, e.g., fluorosilicic acid, H2SiF6). Phosphorus, like nitrogen, is a group 5A element with 5 valence electrons. As expected, phosphorus forms sp3 hybridized compounds with 3 substituents. Simple examples include phosphorus tribromide (PBr3) and trimethylphosphine [(CH3)3P]. Phosphorus forms some compounds in which it has double bonds to oxygen, e.g., phosphoric acid (H3PO4). However, phosphorus’ large size makes p-orbital overlap for p bonds less effective than in C or N compounds. Like other 3rd period elements, phosphorus can be bonded to 4, 5, and 6 atoms. e.g., phosphorus oxychloride (Cl3P=O), phosphorus dibromide trichloride (PBr2Cl3), and phosphorus hexafluoride anion (PF6-). Sulfur, like oxygen, is a group 6A element with 6 valence electrons. As expected, sulfur forms sp3 hybridized compounds with 2 substituents. Simple examples include dimethyl sulfide [(CH3)2S] and methyl mercaptan (methane thiol) (CH3SH). Sulfur can form bonds to three (H2SO3), four (H2SO4), five (SOF4) and six atoms (SF6). A methyl cation has an sp2 hybridized carbocation with a vacant p orbital. A methyl radical has an sp2 hybridized carbon atom with a ½-filled 2p orbital. A methyl anion contains an sp3 hybridized carbanion with a lone pair in one of its sp3 orbitals. Draw them. ORGANIC CHEMISTRY INTRO 27 In saturated compounds, all atoms have only s bonds, whereas in unsaturated compounds, one or more p bonds are present. Conjugated unsaturation occurs when alternating s and p bonds are present. In such compounds, all p-orbitals in conjugated p bonds overlap. 1,3-butadiene CH2 CH H CH2 H H O C C C CH H C H H H H H C C C 2-cylcopentenone H C H H Isolated unsaturation occurs when p bonds are separated by more than one s bond. In such compounds, p-orbitals of one p bond cannot overlap with p-orbitals of other p bonds. CH2 1,4-pentadiene CH CH2 H O C H C C H C H H H H H H 3-cylcopentenone C C H C C H CH2 H H C CH H C H H Cumulated unsaturation describes immediately adjacent unsaturation. Cumulated carbon-to-carbon compounds are not very stable and are rarely encountered. 1,2-butadiene ORGANIC CHEMISTRY INTRO H2C C CH CH3 28 HYBRIDIZATION STATE IS BASED ON THE NUMBER OF REGIONS OF ELECTRON DENSITY Be sp H Be C B .. O .. H N .. O .. C C H C H H C H H C H .. HO .. .. O .. .. O .. .. O H .. - H + N C B H H .. OH .. .. O H H H C H H H .. O N H H H H H NH4+Cl- Si + + H Al : H H H Na+BH4- Mg : .. H O H H sp3 : -: .. : O N - H .. F : .. N .. N + sodium azide H .. O.. H .. O .. Na+ N N H H C .. N .. C B sp2 H O hydronium ion P Cl S sp H H H Al sp2 .. H P C Si H H H CH2 :O : :O : .. S H3C S :O : :O: :O : :O : :O : S :O : - H OH sp3 HO HO Al H H H + Li AlH4- HO HO Si HO HO P Cl .. ..O HO :O : .. Cl S P Cl .. O .. :O : OH OH Cl .. Cl : .. HO HO Cl Cl ORGANIC CHEMISTRY INTRO .. .O . .. ..O : : HO :O : .. Cl S : : HO 29 : Study the following table. In the last 3 columns Lewis structures are drawn as if the atoms were bonded. Learn these names and structures and identify their hybridization states. Lewis Symbol # valence # # bonds unshared e- 's e- 's + 1 F.C. neutral - 1 F.C. B B 3 3 0 - B none boride ion .. C + C- C C 4 4 0 carbonium ion N N 5 3 2 carbide ion 2 4 N N : .. .. O : F 7 1 6 Cl + : 7 1 6 .. .. F .. .. F .. : .. fluoronium ion Cl .. an oxide ion .. F+ O- : oxonium ion : - nitride ion O+ 6 .. nitronium ion .. O .. + .. chloronium ion unhybridized .. .. .. Cl unhybridized unhybridized fluoride ion .. .. Cl : .. unhybridized chloride ion Bromine and iodine are analogous to fluorine and chlorine. Draw the structures of bromonium and iodinium cations, bromide and iodide anions, and bromine and iodine. ORGANIC CHEMISTRY INTRO 30







