Set3
advertisement
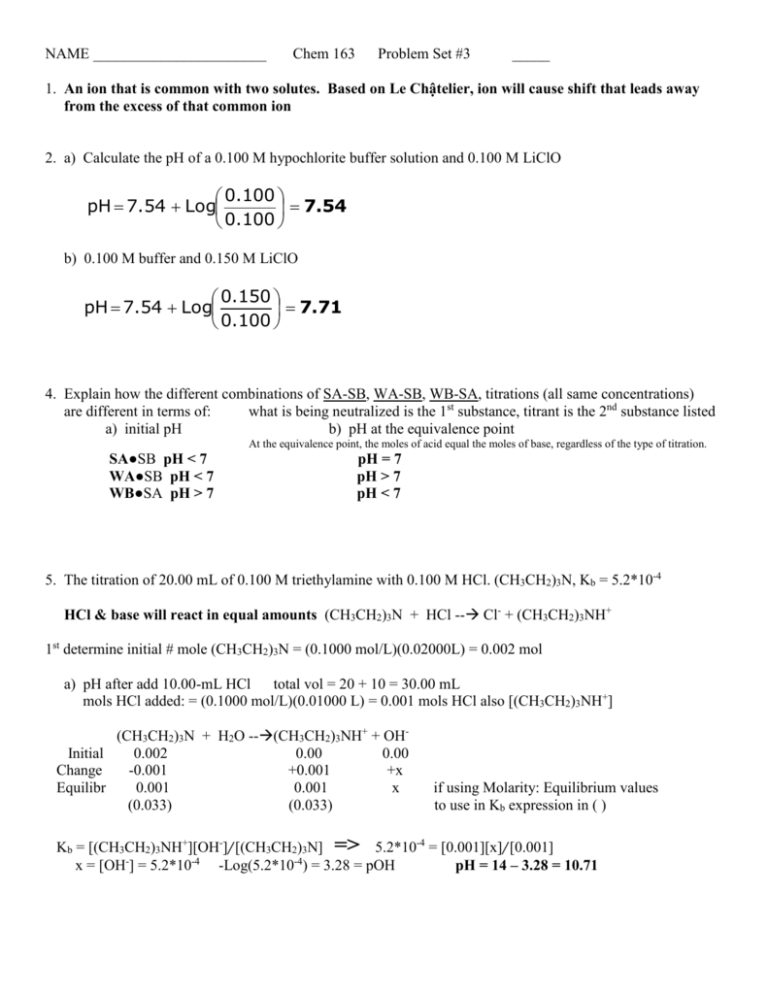
NAME _______________________ Chem 163 Problem Set #3 _____ 1. An ion that is common with two solutes. Based on Le Chậtelier, ion will cause shift that leads away from the excess of that common ion 2. a) Calculate the pH of a 0.100 M hypochlorite buffer solution and 0.100 M LiClO 0.100 pH 7.54 Log 7.54 0.100 b) 0.100 M buffer and 0.150 M LiClO 0.150 pH 7.54 Log 7.71 0.100 4. Explain how the different combinations of SA-SB, WA-SB, WB-SA, titrations (all same concentrations) are different in terms of: what is being neutralized is the 1st substance, titrant is the 2nd substance listed a) initial pH b) pH at the equivalence point At the equivalence point, the moles of acid equal the moles of base, regardless of the type of titration. SA●SB pH < 7 WA●SB pH < 7 WB●SA pH > 7 pH = 7 pH > 7 pH < 7 5. The titration of 20.00 mL of 0.100 M triethylamine with 0.100 M HCl. (CH3CH2)3N, Kb = 5.2*10-4 HCl & base will react in equal amounts (CH3CH2)3N + HCl -- Cl- + (CH3CH2)3NH+ 1st determine initial # mole (CH3CH2)3N = (0.1000 mol/L)(0.02000L) = 0.002 mol a) pH after add 10.00-mL HCl total vol = 20 + 10 = 30.00 mL mols HCl added: = (0.1000 mol/L)(0.01000 L) = 0.001 mols HCl also [(CH3CH2)3NH+] (CH3CH2)3N + H2O --(CH3CH2)3NH+ + OHInitial 0.002 0.00 0.00 Change -0.001 +0.001 +x Equilibr 0.001 0.001 x (0.033) (0.033) if using Molarity: Equilibrium values to use in Kb expression in ( ) Kb = [(CH3CH2)3NH+][OH-]/[(CH3CH2)3N] => 5.2*10-4 = [0.001][x]/[0.001] x = [OH-] = 5.2*10-4 -Log(5.2*10-4) = 3.28 = pOH pH = 14 – 3.28 = 10.71 b) pH after add 19.00-mL HCl total vol = 20 + 19 = 39.00 mL mols HCl added: = (0.1000 mol/L)(0.01900 L) = 0.0019 mols HCl also [(CH3CH2)3NH+] (CH3CH2)3N + H2O --(CH3CH2)3NH+ + OHInitial 0.002 0.00 0.00 Change -0.0019 +0.0019 +x Equilibr 0.0001 0.0019 x (0.00256) (0.0487) if using Molarity: Equilibrium values to use in Kb expression in ( ) Kb = [(CH3CH2)3NH+][OH-]/[(CH3CH2)3N] => 5.2*10-4 = [0.0487][x]/[0.00256] x = [OH-] = 2.73*10-5 -Log(2.73*10-5) = 4.56 = pOH pH = 14 – 4.56 = 9.44 6. Write the ion-product expressions for: a) iron III hydroxide Fe(OH)3 (s) ↔ Fe+3(aq) + 3 OH-(aq) b) barium phosphate Ba3(PO4)2 (s) ↔ 3 Ba+2(aq) + 2 PO4-3(aq) SnS(s) + H2O (l) ↔ Sn+2(aq) + HS-(aq) + OH-(aq) c) tin II sulfide Ksp = [Fe+3][OH-]3 Ksp = [Ba+2]3[PO4-3]2 Ksp = [Sn+2][HS-][OH-] 7. Determine the molar solubility of Ag2SO4 in 0.22M Na2SO4 Ag2SO4 ↔ 2 Ag+ + SO4-2 Initial — Change — Equil — 0 +2x 0.22 +x 2x 0.22+x @ equilibrium [SO4-2] = 2[Ag+] make the assumption that 0.22 + x 0.22 look up Ksp = 1.5*10-5 Ksp = [2Ag+]2[SO4-2] = (2x)2(0.22) 1.5*10-5 = (4x2)(0.22) 1.5 *10-5 x 4.1*10-3 molar solubility of Ag2SO4 4(0.22) 8. Identify, circle, the more soluble compound in water for each pair listed. a) strontium sulfate or barium chromate SrSO4 Ksp: 3.2*10-7 > 2.1*10-10 b) calcium carbonate or copper II carbonate CaCO3 Ksp: 3.3*10-9 > 3*10-12 c) barium iodate or silver chromate Ksp: 1.5*10-9 > 2.6*10-12 BaI2