Evaporation and Boiling
advertisement

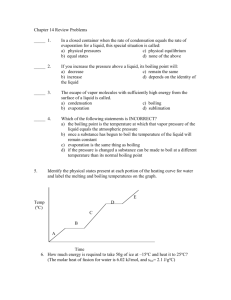
IDS 102 Evaporation and Boiling From past IDS groups we have found that there has been some confusion about evaporation and boiling. Visit the web page below: http://hyperphysics.phy-astr.gsu.edu/hbase/kinetic/vappre.html Where does evaporation happen? What determines whether a water molecule will remain in the water or evaporate and become vapor? Describe the difference between evaporation and boiling. What determines the boiling point of a liquid? If you camp at the top of Mt. Rainier, you may discover that it takes longer to boil potatoes than it would have if you used the same gas stove in a campground at the base of Mt. Rainier. Explain. (check with an instructor before you continue) Cloud Formation Water vapor is a colorless/clear gas—we cannot see water vapor as a true gas when it is in a molecular size. For reasons beyond the level of this course, water condenses more readily if there is something to condense on. The atmosphere also contains compounds called “Cloud Condensation Nuclei”. These very small particles of water-loving substances, such as sea salt, provide a spot for water vapor to condense. As water starts to condense on the CCN, the water droplets get large enough for us to see them due to the reflection of light. The winds in the atmosphere are usually strong enough to keep these larger droplets suspended in the air and as they run into more and more water vapor, the droplets increase in size. Since this process is happening with many other water droplets, there tend to be a bunch in one area and we call these clouds. If there is enough moisture in the cloud and the temperature is low enough for continued condensation to occur, the droplet may reach a size large enough to fall as a raindrop. See the table below for some values of water droplets: Diameter (micrometers-m) 0.2 20 Terminal velocity (m/sec) 0.0000001 0.01 Terminal velocity (ft/sec) 0.0000003 0.03 Type of particle Condensation nuclei Typical cloud droplet 100 0.27 0.9 Large cloud droplet 200 0.7 2.3 Drizzle 1000 4.0 13.1 Small raindrop 2000 6.5 21.4 Typical raindrop 5000 9.0 29.5 Large raindrop From: Ahrens, 1994, Meteorology Today, Fifth Edition, West Publishing (page 194) Determine how many typical cloud droplets there are in a typical raindrop (the volume of a sphere is 4/3 r3). Let’s review something from the pressure module that we recently finished. When you pumped the air out of the plastic jar, what happened to the temperature inside the jar? If air moves up in the atmosphere, it will experience both a reduction in pressure (because there is less air above it) and lower temperatures. So, if air in the atmosphere decreases in temperature, what happens to the water vapor in the air? Let’s think about a parcel of air at sea level in Seattle. The parcel consists of a collection of gases, including water vapor. As the air moves to the east, the air rises to go over the Cascade Mountains. What happens to this air? Draw a picture and explain.