pH Basics
advertisement

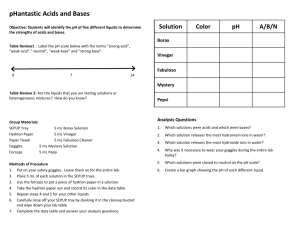
pH Basics This activity will allow your students to explore pH. Although they are already familiar with different substances and their properties based on pH (dangerous chemicals in the house), they probably don’t know why these chemicals are dangerous. This activity will provide them with basic knowledge of pH, how we measure it, and the significance of those numbers for different applications. This activity will also provide the necessary background knowledge for learning about acid rain. Materials required: pH strips or pH meter solutions for pH station – use a selection of 5-6 solutions of acids and bases o lemon, lime or any fruit juice--acidic o soda pop—acidic o instant coffee—acidic o vinegar—acidic o toilet bowl cleaner--very acidic**may be too hazardous (use your discretion) o baking soda in water--basic o table salt in water--should be close to neutral, but may be slightly basic o hand soap--basic o any shampoo, conditioner, detergents--basic o tap water vs. spring or distilled water--on a public system tap should be buffered around 7.3; if from a well could be different (posing a good question to students!) o bleach--very basic**may be too hazardous (use your discretion) plastic cups for solutions (one/solution) labels for each of the cups scrap paper or paper towels for students to lay the used pH strips on safety goggles pH Basics handout (included) Procedures 1. Set up the solutions before the class begins. Use six different solutions of acids and bases. Label each cup. (If students have already learned about pH, don’t label the cups and see if they can guess what they substance is based on the pH!) Use at least six, including two different sources of water. 2. Go over the directions and requirements with your students. Remind them to wear safety goggles. Give a demonstration on proper use of pH strips or meter. 3. Discuss what they found and what this means to them and the things they use. Have they heard anything in the news about acids or bases? How they interpret these stories now? pH Basics pH is the concentration of [H+] ions in a solution concentration. It is a logarithmic scale from 1-14. This means that instead of going up or down by a count of 1, as in math, going up or down one number on the scale increases or decreases by an amount of 10. For example, 2 is 10x more concentrated than 1; 3 is 100x more concentrated than 1. pH 7 is a neutral solution. What is a substance that has a pH of 7? Water. Scientists use pH to determine the acidity of solutions. Solutions below 7 are considered “acids” while those above are “bases.” Where a solution falls on this scale tells us a lot about properties of it and what uses there are for it. Can you think of any acids around your house? (Battery contents, plant fertilizers, vinegar, and inside your stomach!) There are probably many bases around your house as well. (Tums and cleaners.) Many acids and bases are hazardous, so we must use caution with them; others are not…this depends where they fall on the pH scale. How do we know if these solutions are acids are bases? Can you smell it? Sometimes. Is that a good idea? Not usually. Can you taste it? Perhaps. Is that a good idea? Definitely not! Scientists have devised a better, less dangerous way of testing for pH: pH strips. In this activity, you will be testing the pH of a variety of solutions. Do NOT smell or taste any of the solutions. You MUST wear safety goggles during this activity. Fill out the table as you go through the activity. If you have time answer the questions; otherwise wait until you are finished with all of the stations to answer them. Name of solution Characteristics pH Acid or Base? 1. Of the solutions that are acids, what are they used for? 2. Of the solutions that are bases, what are they used for? 3. How do you explain the difference in pH between the two waters?