Biomolecules for Honors Biochemistry II
advertisement

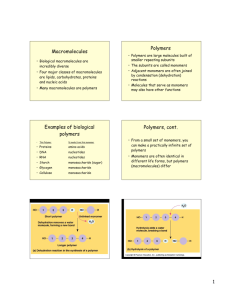
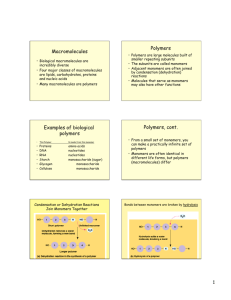
Structure and Function of Macromolecules from Campbell's Biology, Benjamin/Cummings Publishing Co., 1990. Polymers Carbohydrates Lipids Proteins Nucleic Acids Enzymes HW Page Cells can combine small organic molecules into large macromolecules, forming a higher level in the biological hierarchy of biological order. Carbohydrates, lipids, proteins, and nucleic acid are the four major classes of organic compounds in cells. Polymers Macromolecules are polymers, chains of identical or similar subunit molecules called monomers. Although there is a limited number of monomers common to all organisms each organism is unique because of the specific arrangement of these monomers into polymers with distinctive structures and properties. Monomers of all four classes of macromolecules form larger molecules by dehydration synthesis, a chemical reaction in which one monomer donates a hydroxyl group and the other a hydrogen atom, forming a water molecule Polymers can be disassembled to monomers by the reverse process, called hydrolysis. In this way, large macromolecules in food are digested into monomers small enough to enter our cells. return to top Carbohydrates Carbohydrates are sugars and their derivatives. Monosaccharides are the simplest carbohydrates, used directly for fuel, converted to the other types of organic molecules, or used as a monomer units for carbohydrate polymers. All monosaccharides possess a carbon skeleton of three to seven carbons, all but one of which are bonded to a hydroxyl group. The remaining carbon is part of a carbonyl group, and depending on the location of this group the monosaccharide is either a ketone or an aldehyde. In aqueous solution, most monosaccharides form rings. Disaccharides consist of two monosaccharides monomers connected by a glycosidic bond. The monosaccharides can be the same or different. Polysaccharides may consist of thousands of monosaccharide monomers connected by glycosidic bonds. Starch in plants and glycogen in animals are both storage polymers of glucose. Cellulose is an important structural polysaccharide in the cell walls of plants. return to top Lipids Lipids make up the most structurally heterogeneous class of macromolecules, but all share the the property of being wholly or partly insoluble in water Fats are high energy, compact storage molecules also known as triacylglycerols or triglycerides. They are constructed by joining a glycerol molecule to three fatty acids. Fatty acids consist of a carboxyl group and a hydrophobic hydrocarbon tail. The carboxyl group takes part in an ester linkage with the glycerol. Saturated fatty acids have the maximum number of hydrogen atoms because of single bonding between all the carbons. Unsaturated fatty acids (present in oils) have one or more double bonds between the carbons, causing kinks in the molecule and reducing the number of bonding sights for hydrogen atoms. Phospholipids substitute the third fatty acid of a triacylglycerols with a negatively charged phosphate group, which may be joined, in turn, to another small molecule. Such bonding introduces polarity and hence water solubility to one end of the molecule, making phospholipids ideally suited for construction of cell membranes. Steroids, such as cholesterol and the sex hormones, have a carbon skeleton composed of four fused rings, with variation in the number and type of functional groups or atoms attached. return to top Proteins Proteins are polymers constructed from 20 different amino acids. They are the most complex and versatile macromolecules, with emergent properties arising from their intricate architecture. An amino acid is composed of a central asymmetrical carbon singly bonded to a hydrogen atom, a carboxyl group, an amino group, and a variable side chain that confers unique properties on each amino acid. The carboxyl and amino groups of adjacent amino acids link together in a peptide bond, forming long polymers. Protein conformation can be described by three or four superimposed, hierarchical levels. Primary structure is the first level and describes the unique sequence of amino acids. Secondary structure describes how the primary is folded into particular, localized configurations, the alpha helix and beta pleated sheet, which result from hydrogen bonding between peptide linkages. Tertiary structure describes the additional, less regular contortions of the molecule caused by the involvement of side groups in hydrophobic interactions, hydrogen bonds, ionic bonds, and covalent bonds called disulfide bridges. Proteins made of more than one polypeptide chain also show a specific arrangement of their constituent subunits in a quaternary level of structure. The function of a protein is a emergent property of its conformation, which is highly sensitive to conditions such as pH, salt concentration, and temperature. Changing these conditions can cause the protein to denature by altering its shape in such ways that it no longer has a biological function. Protein shape is ultimately determined by its primary structure. Molecular biologists are looking for rules that will predict protein folding and final conformation from an amino acid sequence. return to top Nucleic Acids Nucleic acids are polymers consisting of nucleotides, complex monomers consisting of a pentose (five-carbon sugar) covalently bonded to a phosphate group and to one of five different kinds of nitrogenous bases. In the formation of a polynucleotide, the pentose of one nucleotide joins to the phosphate of another in a phosphodiester linkage, forming a sugar-phosphate backbone from which the nitrogenous bases project. The two kinds of nucleic acids, deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), are named on the basis of their characteristic pentoses: deoxyribose in DNA, and ribose in RNA. The five nitrogenous bases are members of two families, the purines and the pyrimidines, distinctive ring skeletons of carbon and nitrogen with various attached groups. The pyrimidines consist of cytosine (C) thymine (T) and uracil (U); the purines consist of adenine (A) and guanine (G). RNA is a single-stranded nucleotide polymer containing the bases A, G, C, and U. DNA is a helical, double-stranded polymer with bases A, G, C, and T projecting into the interior of the molecule. A always hydrogen bonds to T, and C to G. Thus, the nucleotide sequence of the two strands is complementary, and one strand can serve as a template for the formation of the other. The property of complementary strands give DNA its unique ability to replicate itself and provides a mechanism for the continuity of life. Once replicated specific nucleotide segments of the DNA (genes) program the manufacture of an organism's characteristic proteins. RNA functions in protein synthesis. return to top