Mr Pearson/Ms Tolbert Chemistry Semester 2 – School Year: 2012
advertisement

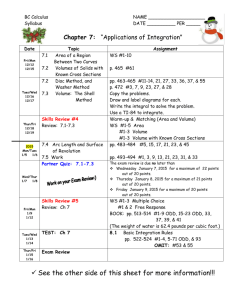
Mr Pearson/Ms Tolbert Chemistry Semester 2 – School Year: 2012-2013 Room 009 01/21/13 01/22/13 01/23/13 01/24/13 01/25/13 Mon Tue Wed Thur Fri MLK Holiday- No School Introduction to Solubility using Data Chart Lab: Solubility of an Unknown Compound Solubility – the big picture Solubility Rules Monday 1/21: No School – MLK Day Tuesday 1/22: Learning Target: Students will be able to translate observations from their data chart onto a Solubility table, and begin to observe trends. - Discussion about Lab Final - New Seating Chart - Solubility Worksheet – use Data Table (from final) to complete - Solubility reading in your textbook, Chapter 14: o p.476-479 (should be a review) o p.492-494 Wednesday 1/23: - Thursday 1/24: - - Learning Target: Work together as a class to determine saturation points (precipitation point) of an unknown Compound. Go into lab, record data for the concentration of the unknown for your group Solubility reading in your textbook, Chapter 14: o p.476-479 (should be a review) o p.492-494 Learning Target: Students will be able to compare Unknown Compound (determined with class data) to an existing Solubility Curve to determine the unknown. Prezi about Solubility – take notes – also available on website Share lab results, enter into data table, make a graph Solubility Curves and our unknown o Distribute Solubility Curve Questions Worksheet (use when writing conclusion) Solubility reading in your textbook, Chapter 14: o p.476-479 (should be a review) o p.492-494 - Write a conclusion using new vocab and graph data to explain how we determined the unknown compound for the lab and how it compares to the established values for this compound. Friday 1/25: Learning Target: Use solubility rules to guess which compound will readily dissolve in water: Copper (II) Hydroxide or Copper (II) Nitrate. - Discuss Solubility Curves worksheet - Conclusion for Unknown Compound Lab due Monday o Only 1 conclusion needed for lab partners (do it together!) o Staple the class data table to your conclusion o Refer to website as necessary, to view prezi or download Excel file Excel files can be opened in Google Drive, with a google account - Class time to work on Soluble/Insoluble worksheet due Mon (first assignment of 3rd quarter), the Lab Conclusion, and Solubility Curves worksheet due Wed 01/28/13 01/29/13 01/30/13 01/31/13 02/01/13 Mon Tue Wed Thur Fri Molarity, molality How Low Can You Go? (concentration lab) Colligative Properties Concentration Predicting Chemical Reactions (nomenclature, balancing and predicting) Monday 1/28: Learning Target: Students will be able to calculate the Molarity of a solution made with 84.8g of LiCl. - Distribute M&m Worksheet - Conclusion for Unknown Substance due (1 conclusion for each set of lab partners – staple it to your table with all of the class results listed) - Solubility Table worksheet due Tuesday 1/29: Lab – How Low Can You Go? Wednesday 1/30: Thursday 1/31: - Learning Target: Students will be able to anticipate the Molarity of a solution when 2 moles of NaCl are mixed into a solution to a final volume of 1,317mL. Solubility Curve worksheet is due Discuss M&m worksheet Practice mixing Molarity solutions Learning Target: Students will be able to calculate the boiling point elevation for 15g of sucrose (C12H22O11) in 250g of water. Additional time to work on M&m worksheet Colligative Properties (on M&m and lab) Friday 2/1: (pep fest schedule) Learning Target: Students will be able to predict the products of a - reaction, and which substance is likely to precipitate out. Distribute Mr Jagush’s Predicting Chemical Reactions worksheet: Due Tuesday 2/5 with the temp lab Physically create molecules with student bodies (water, NaCl, CaCl2) (6th period ends at 1:25) 02/04/13 02/05/13 02/06/13 02/07/13 02/08/13 Mon Tue Wed Thur Fri Colligative Properties and Chemical Reactions Test Review Day Test – Solutions and Solubility (M, m, colligative properties, solubility) Lab – Organizing compounds by behavior in Lab Ionic and Covalent Bonds Monday 2/4: Learning Target: Students will be able to determine how many grams of ethanol (CH3OH), must be dissolved in 500 grams of water to lower the freezing point to -6.51oC. - M&m stamped for completion (collected on Test Day) Tuesday 2/5: Solutions and Solubility Test Review - H.L.C.Y.G. Lab due - INDIVIDUALLY Complete the Review Sheet, see Teacher to check answers and be dubbed a “sage” o Sages get an answer sheet and begin to help other students Wednesday 2/6: - Solutions and Solubility Quiz TEST Predicting Chemical Rxn’s due Turn in M&m sheet for a grade (stamped as on-time on Monday) Thursday 2/7: Learning Target: Each group will organize six different compounds based on their behavior in lab – based on observations to one of three experiments. Nature of Science, making sense of a mixed up world Turning observations into organized groups or a ranked series Prepare to share an explanation of groupings with the class on Monday Monday: Data will be combined and students will look for trends Careful of contamination, toxicity, use tools to enhance your basic procedure Friday 2/8 Learning target: Take previous knowledge of atomic valence shells and start using a shorthand to demonstrate valence electrons – via the electron dot model. Be able to draw a model for a (neutral) aluminum atom. - Review valence shells - Hund’s rule - Introduce dot model: green handout of notes - Practice using dot model (white worksheet due Tuesday) 02/11/13 02/12/13 02/13/13 02/14/13 02/15/13 Mon Tue Wed Thur Fri Monday 2/11: - Tuesday 2/12: - Wednesday 2/13: - Thursday 2/14: - - Lab share; grouping types of materials (kinds of bonds) Mixed electron dot practice packet: multiple bonds; “coordinate bonds” 3-D molecular models Polarity and Electonegativity Friday Five Quiz, Electronegativity Learning Target: Students will find trends in their cumulative lab data and elaborate on the reason for those trends. Review, electron dot model (from Friday, worksheet due Tues) Share lab data (from Thursday) put in Spreadsheet TPS about class data Electron glue in Cl2, CH4, NaCl Test grades are in the portal, come in after school to see your test and/or retake it **Hold on to Your Lab Data** You will be assigned to write a conclusion as we learn more about what you discovered on Thursday. Learning Target: Students will be able to illustrate in 2D electron Common mistakes on the Solubility, Concentration Test o Reminder: see/retake your test after school Electron Dot Model sheet is due Discuss mixed ions sheet, introduce multiple bonds (due Tues) Chapter 8, p.240-243 **read/practice problems about covalent and ionic bonds** Lab: Students will discover the best way to arrange atoms in molecule / formula unit. Ball and stick model for bonding (due Tues) Polarity/ VSEPR and vocabulary note sheet Chapter 8, p253-257 and p261-264 **read/practice problems about molecular structures and VSEPR models** Learning Target: Polarity, Electronegativity and Polyatomic Ions Chapter 8, 265-270 **read/practice problems about polarity and electronegativity** o You should have a golden, Electronegativity Table already, see p. 265 for a table in your book Demonstrate HNO2 and NO3-1 Tuesday students will present Electron dot solutions on the Smart board and white board Friday 2/15: Learning Target: check understanding of bonding and shape basics; get more practice with Dot structures and 3-D understanding of shape and polarity - Friday Five Quiz last 10 minutes of the hour - Electronegativity from ACT prep (due Wed) 02/18/13 02/19/13 Mon Tue No School - Presidents Day Revisit Polarity, Electronegativity, Bonding (Mixed electron dot worksheet and 3-D modles of molecules polar or non-polar DUE) 02/20/13 Wed Lab – Like Dissolves Like; (ACT worksheet checked) 02/21/13 Thur Test Review 02/22/13 Fri Test Monday 2/18: No School – Presidents Day Forgot to give 6th hour the ACT practice (think I gave to 5th hour; 4th got for sure) Tuesday 2/19: Learning Target: - Students will draw molecules on smartboard and whiteboard by drawing Electron dot or VSEPR 3D models - Due today the 3-D models lab; the mixed electron dot practice o WRITE down (then we will share) how you go about drawing and then thinking about these dot structures o Share examples: (any from the worksheet: 11) Cl2, 20)CO2, 21) HClO4 , 25) HCN , 27) C2H2 , 41) H3O+1 any others What types of bonds, is the molecule polar? Wednesday 2/20: Lab – Like Dissolves Like - 2 solvents, 7 solutes identify behavior and classify “type” Thursday 2/21: - Friday 2/22: - Learning Target: Review, bonding, polarity, IMF’s Take out the Electronegativity “ACT practice questions” we were going to check yesterday but forgot (if you turned in () pick it back up) Like Dissolves Like Lab – due Monday o turn in 1 per 2 person group – that means 2 per lab bench Get back the Friday 5 quiz; We have a practice/review sheet to help you study for tomorrows test (Yes, you can have hand written notes for the test) o Be able to do more than just draw the electron dot models; Shape, polarity, type of bond, etc Learning Target: MEGA TEST (yes, you can use hand written notes) Conclusion for Group Lab due (melting, dissolving or conducting; info on website) Like Dissolves Like Lab due Mon Test has 2 parts: o 30 true/false & MC questions on a scantron sheet; (ID A or ID B) - 02/25/13 02/26/13 02/27/13 02/28/13 03/01/13 o one side of a free response page make sure you put name on BOTH and say which version of the questions you answered ID A or ID B reading in Chapter 12 next; states of matter; Phase changes and thermo chemistry. NO3-? Means NO3-1 ? Mon Tue Wed Thur Fri Phase of matter and Phase transitions; q = mhfus q = mhvap Phase Diagrams and Heating Curves q = mCT; “C” is the specific heat of the material; CH2O = 1 cal/goC Specific heat of metal lab No School conferences – Remind your parents Monday 2/25: Learning Target: - Polar Molecules – IMF - Drops of water on a penny - Look at Prezi; read in text (411-419) - Missing assignments Tuesday 2/26: Learning Target: - Science probe, show video: http://www.youtube.com/watch?v=cOYl1raXPz8 o Students will write what they think the temperatures will be with a vigorous, rolling boil vs a simmer - Temperature scales (show comparisons for 0°F, 32°F, 98.6°F, 212°F) o Distribute Temperature conversion worksheets - Demonstration of heating icewater to boil (time and temp monitored) on heating stir plate w/ vernier probe o start with 400mL of icewater – or less o You may have to restart Logger Pro (now on start menu) - Phase changes diagrams, triple point etc on Prezi o Prezi: http://prezi.com/6rtrjuugvkw_/matter/ - Heating curves, plateau at Hfusion, Hvapor o Look at plateau’s on vernier data - Show Heating simulation from class website o http://southwest.mpls.k12.mn.us/Pearson - Discuss Specific Heat Q=mcT o Distribute Heating Curves worksheet - Read p. 519-522 (also 411-419 if you didn’t read that last night from the Prezi you saw on phases and phase changes) Wednesday 2/27: Learning Target: What effect does adding heat have? ****LAST DAY TO RETAKE SOLUBILITY/M&m TEST**** - Phase changes diagrams, triple point etc on Prezi o Prezi: http://prezi.com/6rtrjuugvkw_/matter/ - Heating curves, plateau at Hfusion, Hvapor o Look at plateau’s on vernier data Thursday 2/28: - Show Heating simulation from class website o http://southwest.mpls.k12.mn.us/Pearson Discuss Specific Heat Q=mCT o Distribute Specific Heat practice sheet (due Tuesday) Change mass, how does the quantity of heat required change? Remind your parents that conferences start tomorrow night (also during the day on Friday) If time: Discuss tomorrow’s specific heat lab. Learning Target: Lab – Specific Heat Lab Keep your Temperature conversion problems on your desk while you work on the lab (so I can check them off) Two people per Lab sheet will be okay o We will transfer ENERGY (calories) to the room temp water; the energy will be a part of the metal we use to convey this energy. Remember that the specific heat of water is Cwater = 1.0 calorie/goC o We will then calculate the specific heat (Cmetal) of the material once we have determined how much energy the metal gives up. NO SCHOOL Conferences Friday 3/1: 03/04/13 03/05/13 03/06/13 03/07/13 03/08/13 Mon Tue Wed Thur Fri Monday 3/04: Tuesday 3/05: - - Energy of breaking/Making Bonds Calorie is a ratio, energy exchange diagrams, Clicker quiz Calorimetry Lab - Peanut Gummy Bear Demo, Activation Energy Hess’ Law, in-depth reaction calculations Learning Target: Heat curves sheet is due, get it out, we’ll come around and check it for completion Reading: p. 523-533 Annotating for Specific Heat worksheet Endothermic vs Exothermic Energy needed to break bonds, energy released when making bonds o Different bonds have different energy Thermite Demo Energy exchange diagram Learning Target: Reading: p. 523-533 (chapter 15) Specific Heat problems due o Answers posted on website, videos for #3, #7 on website o One problem from this sheet will be on the test Calorie is a derived unit (ratio) o Calories/mol o Consider chips and serving size - Demo: NH4NO3 + H2O ??? Demo: CaCl2 + H2O ??? energy exchange diagrams (put heat on appropriate side of rxn) Clicker Quiz Wednesday 3/06: Lab: Burning Peanut – Calorimetry lab - If you are allergic to peanuts let me know NOW - Specific Heat Lab due today - Get lab handout: each person gets one but if you wish you can turn in with a partner o Use the electronic scales to save time but to avoid the line you can use the triple beam balances o The burning peanut will heat the can and the water inside (and what else? --> leading to errors) so you need to know the mass of each item we know that water has a density of 1 g/ml so we can use a graduated cylinder to measure out the mass of water we are adding to the can 46.5 ml (of pure water) = 46.5 grams (of pure water) o The chemical reaction is a partial combustion of the peanut (mixture of mostly proteins and fats) which is why you see so much soot (not enough oxygen to turn all the carbon into carbon dioxide) o You have to add enough water (and use a small enough peanut sample) that we don’t get it boiling or there is more math in the calculations o Have fun, stay safe, learn lots Thursday 3/07: - - Friday 3/08: - Learning Target: Students will be able to draw an energy exchange diagram for the combustion of a gummy bear. Blast Balls demonstration o Draw energy exchange diagram for endo/exothermic reactions. Show student work on document camera o Why didn’t the thermite and blast balls react right away? Distribute sheet with Chemical Reaction Energy Diagram, label diagram Determine energy from nutrition information about a gummy bear? o Due Monday Gummy bear demonstration Burning sugar cube (if time) Read p. 564 – 565 o Next Monday we’re going to start working on presentations based on reading, make sure you’ve done all the assigned reading – pages: 411-419 519-522 523-533 564-565 Learning Target: Heat of fusion/vaporization review Specific Heat problems that include phase changes o Burning sugar cube demo - 03/11/13 03/12/13 03/13/13 03/14/13 03/15/13 Catalyst affect on energy diagrams o Answer #6 on gummy bear worksheet Hess’s Law Assigned reading and homework problems o Read p.523-528, do practice problems #16-19, #22 **reading was assigned on Tuesday, only the problems are new o Next Monday we’re going to start working on presentations based on reading, make sure you’ve done all the assigned reading – pages: 411-419 519-522 523-533 564-565 Mon Tue Wed Thur Fri Monday 3/11: - Reading/Presentation Prep Test Review w/ Presentations Test Learning Target: Assigned reading, presenting topics o Get in your group, write your name and birthday on the top of your sheet o Read the pages that pertain to your subject o Identify key vocab words o Come up with a relevant real world example o Prepare to present your subject to class Tuesday 3/12: Test Review – participate/take notes on 5 minute presentations on subjects Wednesday 3/13: Test on Energy and Chemical Change Thursday 3/14: Learning Target: Friday 3/15: Learning Target: 03/18/13 03/19/13 03/20/13 03/21/13 03/22/13 Mon Tue Wed Thur Fri pre-ACT test Monday 3/18: Learning Target: Tuesday 3/19: NO CLASS – pre-ACT test Wednesday 3/20: Learning Target: Thursday 3/21: Learning Target: Friday 3/22: Learning Target: 03/25/13 03/26/13 03/27/13 03/28/13 03/29/13 Mon Tue Wed Thur Fri Monday 12/10: Learning Target: Tuesday 12/11: Learning Target: Wednesday 12/12: Learning Target: Thursday 12/13: Learning Target: Friday 12/14: Learning Target: 03/11/13 03/12/13 03/13/13 03/14/13 03/15/13 Mon Tue Wed Thur Fri 03/18/13 03/19/13 03/20/13 03/21/13 03/22/13 Mon Tue Wed Thur Fri 03/25/13 03/26/13 03/27/13 03/28/13 03/29/13 Mon Tue Wed Thur Fri 3rd quarter finals 3rd quarter finals No school for students SPRING BREAK 04/08/13 04/09/13 04/10/13 04/11/13 04/12/13 Mon Tue Wed Thur Fri 04/15/13 04/16/13 04/17/13 04/18/13 04/19/13 Mon Tue Wed Thur Fri 04/22/13 04/23/13 04/24/13 04/25/13 04/26/13 Mon Tue Wed Thur Fri 04/29/13 04/30/13 05/01/13 05/02/13 05/03/13 Mon Tue Wed Thur Fri 05/06/13 05/07/13 05/08/13 05/09/13 05/10/13 Mon Tue Wed Thur Fri 05/13/13 05/14/13 05/15/13 05/16/13 05/17/13 Mon Tue Wed Thur Fri 05/20/13 05/21/13 05/22/13 05/23/13 05/24/13 Mon Tue Wed Thur Fri 05/27/13 Mon NO SCHOOL NO SCHOOL 05/28/13 05/29/13 05/30/13 05/31/13 Tue Wed Thur Fri