File
advertisement

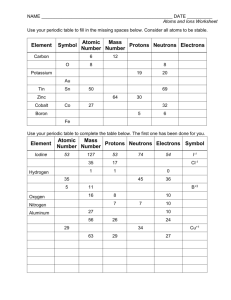
If the number of protons and electrons are NOT the same (unequal), you are left with an _______. Ions are electrically charged because there is a change in the number of _________________. (If you change the number of the PROTONS what happens???) A positively (+) charged ion means _____________________________________________ A negatively (-) charged ion means ______________________________________________ ION CHARGE = Given the information below, determine the charges on the following ions AND write the complete chemical symbol for each. p= 28 e= 26 n= 30 p= 17 e= 18 n= 18 p= 74 e= 72 n=108 p= 8 e= 10 n= 8 What is the complete chemical symbol for the ion with 12 protons and 10 electrons? Write the complete chemical symbol for the ion with 95 protons and 89 electrons. Write the complete chemical symbol for the ion with 33 protons and 36 electrons. 45 How many protons, electrons, and neutrons are present in the 21 Sc+3 ion? Fill in the following table. Assume all are atoms unless there is enough information to determine if there is a charge! (The ONLY WAY you would recognize an ION is if the symbol is shown and there is a charge in the upper right hand corner OR if the # of electrons is DIFFERENT from the # of protons!) Chemical Symbol # of protons # of electrons # of neutrons 53 74 Atom or Ion? 80Br 133 55 54 20 18 23Na+1 75As 20 27 32 56 81 Ag 60 N Complete the following. # p+ 27 13 Al 10 # e- #n0 7 atomic # mass # ion atom or ion +3 -------------------------------------------------------------------------------------79 34 Se -2 Write the complete chemical symbol given the following information. P = 79 E = 76 N= 118 P= 9 E= 10 N= 10