Anna Jonhed,
advertisement

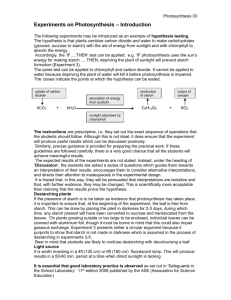
Anna Jonhed, Lars Jarnstrom Department of Chemistry, Karlstad University, Karlstad, Sweden Phase and Gelation Behavior of 2-Hydroxy-3(N,N-dimethyl-N-dodecylammonium)propyloxy Starches Some starches containing quaternary dimethylalkylammonium groups exhibit an unique phase behavior. A solid phase or gel phase is formed upon cooling, i.e. they are temperature-responsive polymers. The aim of this study was to investigate the phase and gelation behavior of hydrophobically modified quaternary ammonium starch ethers in aqueous solutions. The mechanisms behind the phase behavior and hydrophobic character were investigated by light scattering (turbidity) and rheoiogicai measurements. A relatively large increase in the complex viscosity at higher concentrations was observed when the solutions were cooled to room temperature. The phase angle decreased drastically at a certain critical temperature. The decrease in the phase angle depended on the concentration of starch in solution, higher concentrations showing the greatest decrease and lower concentrations showing no significant change. Turbidity measurements indicated that a solid-like highly concentrated phase was precipitated. The starch with zero net charge showed a larger increase in turbidity than the starch with a positive net charge, which indicates that particular precipitation is favored by a zero net charge and that the formation of a gel network is favored by charged starch molecules. Keywords: Modified starches; Gelation; Phase separation 1 Introduction Interest in new "special" grades of starch in paper coating and paper surface sizing is constantly increasing. Examples can be found in new concepts for ink-jet papers, coatings with greater fiber coverage, etc. Several new starch grades are hydrophobically modified. These can be synthesized as anionic [1], cationic [2], or non-ionic [3] derivatives. Two of the most promising and recently most investigated derivatives are the substituted succinate derivatives and the derivatives of epoxypropyldimethyl-alkylammonium chloride. The succinate derivatives have one permanent negative charge attached to each pendant hydrophobic group and the hydrophobic quaternary ammonium derivatives have one permanent positive charge attached to each pending hydrophobic group. Both the succinate and the quaternary ammonium reagents form products that are used in the paper field. Much has been written about the advantages of using cationic starch in papermaking. Starch is added to paper to increase its strength, both in internal and surface sizing [4]. Only a small proportion of an unmodified starch is retained on cellulose fibers in a one-pass retention for inCorrespondence: Anna Jonhed, Department of Chemistry, Karlstad University, SE-65188 Karlstad, Sweden. Phone: + 46-54-7001571, Fax: +46-54-7002040, e-mail: anna.jonhed@kau.se. T emal sizing. A significantly better retention is achieved with cationic starch. The binding power of cationic starch is higher than that of native starch because the ionic interactions of the starch with fiber and fillers are stronger than simple hydrogen bonds. In addition, the greater stability of the molecules of the modified starch and their inherently better rheology mean that they can be used with higher molecular weights without runnability problems [2]. The phase behavior of hydrophobically modified non-ionic polymers has been studied for a long time. Extensive studies have been directed towards understanding the associative behavior of hydrophobically modified cellulose ethers [5] and the phase behavior of mixtures of such cellulose derivatives and surfactants [6]. A diblock copolymer and a polymer with pendant hydrophobic groups may show a self-assembly similar to that of a surfactant. The associative properties of block copolymers and hydrophobically modified polymers have made them useful as thickeners in waterborne suspensions [7]. Beside the micelle-like type of associative behavior, a polymer that shows a coil-helix transition can act as a host molecule in inclusion complexes. This has been reported for amylose in blends with hydrophobically modified cellulose ethers [8]. Many cellulose ethers show a reversible phase separation at higher temperatures. This paper concentrates on starches modified by the quaternary ammonium reagent, containing methyl and dodecyl moieties. These starches are insoluble in water at low but soluble at high temperatures. At high concentrations they form gels, and at low concentrations they form particulate suspensions, as reported elsewhere [9]. It has been shown in technical applications, such as the surface treatment of paper [10], that treatment by the hy-drophobically modified starches investigated here leads to a decrease in the penetration of water and to an increase in the contact angle of a water droplet. This is probably due to the lower solubility in water of the modified starch grades. A main topic of interest is to investigate the role of the hy-drophobic chain-groups and how they affect the phase separation. It is important to understand the mechanism of phase separation in order to be able to improve the use of starch in the surface coating of paper. In the present investigation, turbidity and rheological measurements are used to determine the mechanism behind the phase separation. 2 Materials and Methods 2.1 Materials Two grades of 2-hydroxy-3-(N,N-dimethyl-N-dodecyl-ammonium)propyloxy starch and one oxidized starch were used. All grades were based on native potato starch (supplied by Lyckeby Starkelsen, Kristianstad, Sweden) and the degree of substitution for each starch grade is given in Tab. 1. The hydrophobically modified starches were oxidized in the same way as the only oxidized grade prior to hydrophobization: starch was slurried in distilled water and the temperature was raised to 36 °C. The pH was adjusted to 9.5. Oxidization was performed by drop-wise addition of sodium hypochlorite (technical grade) at pH 9.5 and 36 °C until a colorless sample with potassium iodide was obtained. 1 g of sodium bisulfite per kg starch was added to terminate the reaction. The pH was lowered to 5.5 before filtration, washing and drying. The starch grade obtained by this oxidation is denoted Starch A. The result of the oxidation was determined by potentio-metric titration: 5 g starch was suspended in 25 mL 0.1 M aqueous HCI and agitated for 30 min. The suspension was dewatered and dissolved in 300 mL water. After the suspension had been heated for 10 min at 95 °C, the warm suspension was titrated with 0.1 M NaOH and the result interpreted as DS with respect to carboxylic groups although other oxidation products may exist as well. The results in Tab. 1 were corrected for the presence of phosphate groups. Potato starch contains mo-noester phosphate groups corresponding to DS 0.00436 [1]. The molecular weight distribution of oxidized starch (DSCOOH = 0.03) is very broad and the main fraction varies from about 104 to 106 [11]. The hydrophobic modification was performed by reacting a pre-oxidized starch, Starch A, with a quaternary amine reagent (3-chloro-2-hydroxypropyldimethyldode-cylammonium chloride) at alkaline pH and ambient temperature (pH 11.3 and 37.5 °C). When the reaction was complete, the pH was adjusted to 9.5. In this way one positive charge was introduced per hydrophobic group. Fig. 1a shows the chemical structure of the quaternary amine used. The reaction product was carefully washed with water in order to remove any remaining reagent and intermediates. The degree of substitution of hydropho-bic/cationic groups was determined by analyzing the nitrogen content using the Kjeldahl method. The Kjeldahl analyses were performed by the starch supplier, the error range was estimated by the supplier to ± 4%. The charge densities of Starches B and C were measured using a Particle Charge Detector (Mutek, PCD 03, Herrsching, Germany) at pH 8. The anionic starch was titrated with poly(diallyldimethylammonium chloride) [CAS no. 26062-79-3] [12] and the cationic starch was titrated with polyethylene sodium sulfonate [CAS no. 25053-27-4]. The resulting modified starches are amphiphilic and am-photeric (Fig. 1 b). The characteristics of the starches are summarized in Tab. 1. Fig. 1. a) Chemical structure of quaternary amine used for hydrophobizing starch, b) Chemical structure (schematically) for the hydrophobically modified starch. The positions of the functional groups are chosen arbitrarily. Tab. 1. Degree of modification of various potato starch products. DSN is the degree of substitution with respect to the hydrophobic part, i.e. the carbon chain, and DSCOOH is the degree of substitution with respect to the oxidized part. 2.2 Preparation of starch solutions Starch solutions were prepared by dispersing about 12 g starch in about 100 g distilled water. The solution was heated to 95 °C while being stirred at 300 rpm. The starch suspensions were held at 95 °C for 30 min while stirred at 500 to 600 rpm. If not used immediately they were stored in a sealed container at 80 °C. Each batch was investigated under a light microscope in order to ensure that the starch was fully cooked. The pH was 8 for all the starch solutions. No salt was added to any solution. 2.3 Determination of the overlap concentration The viscosity (η) was measured using a conventional controlled-shear-stress rheometer (Paar Physica, MCR 300, Graz, Austria). Rotational flow curve measurements were made with the double gap geometry, DG 26.7 at 20 °C on Starch A. The shear rate was 10 s-1. The overlap concentration was determined by plotting the viscosity as a function of starch concentration. 2.4 Determination of turbidity The transmittance of the solution was measured on a conventional UV-spectrophotometer (Shimadzu, UV2101 PC, Kyoto, Japan). Each sample was run in a cycle from 80 °C to room temperature and back to 80 °C, with a duration of 1 h for each run. The turbidity (τ) was calculated from the transmittance (T) of a light beam passing through a cell of length (L), according to: 2.5 Characterization by rheology The rheological properties of the starch solutions were measured using a conventional controlledshear-stress rheometer (Paar Physica, MCR 300, Graz, Austria). Amplitude sweeps were performed to determine the linear viscoelastic region. Oscillatory measurements were performed with a concentric cylinder geometry in the linear viscoelastic region. The temperature was varied between 80 and 20 °C, while the amplitude (1) and the frequency (1 Hz) were held constant, in order to determine the storage and loss moduli (G' and G"), the complex viscosity (η*) and the phase angle (8) as a function of temperature. The absolute value of η* was plotted. The ramping time was 60 min for one cooling-reheating loop. The relation between complex viscosity and moduli is given by where G' is the storage modulus, G" is the loss modulus and ω is the frequency of oscillation. The phase angle 8 is defined as: 2.6 Particle size determination The particle size distribution was measured after separation of the precipitate and re-dispersing in water on a Coulter LS130 Fluid Model (Coulter Electronics Ltd. Lu-ton, England) at a starch concentration of about 0.1%. Distilled water was used as fluid medium. To some samples a nonionic surfactant, decanol ethoxylate (Ethylan® 1005 from Akzo Nobel, gplace?g, Sweden), was added during the re-dispersion process in order to make sure that the measurements were performed on the dispersed particles and not particle aggregates. The yield of the precipitation was determined gravimetrically after separation by centrifugation. 3 Results These starches exhibited temperature-responsive properties, i.e. either gelation or phase separation occurred at a certain temperature upon cooling, as detected by turbidity measurements and rheology. 3.1 Overlap concentration When the viscosity of the only oxidized starch grade (Starch A) was plotted vs. concentration, the overlap concentration (c*) was revealed as the onset of a steep increase in viscosity with increasing starch concentration, as shown in Fig. 2. This overlap concentration was determined as c* ≈ 3.5% (w/w) at pH 8. 3.2 Turbidity measurements and particle sizing The solution of Starch B in water showed an increase in turbidity (Τ) with decreasing temperature, indicating a Fig. 2. Viscosity as a function of concentration for starch A, pH=8.0 and 20 °C. phase separation process at low temperatures. At a given temperature, the solution with a lower starch concentration showed a higher turbidity than the solution with higher starch concentration, as shown in Fig. 3. The solution of Starch C showed an increase in turbidity with decreasing temperature, but in this case the solution with a lower starch concentration showed a lower turbidity at a given temperature than the solution with higher starch concentration. The increase in turbidity for Starch C was much weaker than for Starch B. The turbidity of starch A was unaffected by the temperature and this is shown as a reference in Fig. 3. The particle size measurements showed a mean particle size of 20 μm for Starch B. When adding a non-ionic surfactant, the particle size did not change. Since the addition of the surfactant did not reduce the measured particle size, one may conclude that the primary particle size was about 20 μm or that the attractive forces within an aggregate were strong enough to withstand the dispersing effect of the surfactant. For starch solutions with initial concentrations of 10% (w/w), the yield of precipitated particles of Starch B was 15% and for Starch C 4%. 3.3 Rheological measurements Fig. 4 shows the complex viscosity of the starch solutions as a function of temperature measured during a cooling and reheating loop at continuous oscillatory shear. A clear hysteresis loop in complex viscosity was observed at high concentrations of Starch C. The complex viscosity obtained at cooling was always lower than the corresponding value obtained at re-heating. Starch A is shown for comparison. When exhibited to the cooling ramp, the complex viscosity of Starch C started to increase rapidly at about 45 °C, as shown in Fig. 4. Starch B and Starch A showed a lower temperature effect on the complex viscosity. Fig. 5 shows the complex viscosity as a function of concentration for the three starch grades measured when the cooling was allowed to take place at rest without oscillatory shear. It is evident from Figs. 4 and 5 that the hydrophobically modified starch C exhibited a large increase in complex viscosity with increasing concentration. The increase in complex viscosity for Starch B after cooling without oscillatory shear was similar to that observed for Starch A and could be explained as an Arrhenius effect. However, when the starches were Fig. 3. Turbidity as a function of temperature for different starch grades at 618 nm, pH=8. Starch B: 4.7% (w/w) (▲) and 7.6% (w/w) (∆); Starch C: 7.8% (w/w) (■) and 4.6% (w/w) (□); Starch A: 8.2% (w/w) (x) is shown as a reference. The figures show cooling from 80 °C to room temperature. Fig. 4. Complex viscosity as a function of temperature for different starches and concentrations at pH=8. Starch C: 8.9% (w/w) (♦), 7.8% (w/w) (◊), 6.5 %(w/w) (-); Starch B: 7.6 %(w/w) (∆), 6.0% (w/w) (▲), Starch A: 9.2% (w/w) (x). The arrows indicate the temperature loop for Starch C at 8.9% (w/w) %. Fig. 5. Complex viscosity as a function of concentration for Starch A (x), B (■), and C (∆) at 20 °C. Fig. 6. Phase angle, 5, as a function of temperature at pH =8. Starch C: 12.1% (w/w) (∆), 6.5% (w/w) (□), Starch B: 12.2% (w/w) (♦), 7.6% (w/w) (x), 6.0% (w/w) (▲), Starch A: 9.2% (w/w) (●). The arrows indicate the temperature loop for Starch C at 12.1% (w/w). cooled down during oscillation, the complex viscosity at room temperature for Starch B was substantially higher than that of Starch A. The onset temperatures for the drop of the phase angle from 90° are shown in Fig. 6. Starch A is shown as a reference and shows a behavior similar to that of Starch B. For Starch C a clear hysteresis loop in phase angle was observed at high concentrations. The phase angle obtained at cooling was always higher than the value obtained at re-heating, however, the loop was reversible. 4 Discussion Fig. 3 shows the turbidity as a function of temperature. Phase separation of a starch solution creates a turbid system. The scattering of light and the turbidity depend strongly on the particle size. Starch B showed a rapid increase in turbidity, T, with decreasing temperature. At a given temperature, the solution with a lower starch concentration showed a higher turbidity than the solution with a higher starch concentration. Precipitation occurred more readily at low starch concentration, similar to what is observed for retrogradation of unmodified starches where a gel is formed in concentrated solutions and a precipitate is formed in dilute solutions [1]. In all cases, the turbidity measurements showed that the behavior was reversible. Thus, retrogradation cannot be the explanation for the phase separation observed by the turbidity measurements. The mean particle size for Starch B was 20 μm. Järnström et al. [9] reported that the mean particle size of a precipitate formed from solutions of starch similar to Starch B and Starch C was slightly above 1 (im. The bigger size of the precipitate observed in the present investigation indicated that the DS and the rate of cooling affected the precipitation process. The increase in τ was greater for Starch B than for Starch C and the yield of precipitation was determined to 15% for Starch B whereas Starch C only had a precipitation yield of 4%. Starch C has a higher charge density than Starch B, which has a net charge density close to zero, and this may be one possible explanation to the difference in turbidity. A remarkable feature was that introduction of only 3% carbon atoms from the synthetic reagent was required to produce starch derivatives insoluble in water. This is a remarkably low value for water solubility and can be compared to degraded starch acetates that are soluble in water to an acetyl content of up to 25%, corresponding toDS=1.25[1]. Fig. 4 shows that the complex viscosity (η*) of starch B and C increased with decreasing temperature, indicating the formation of a gel network in combination with the normal viscositytemperature behavior of a polymer solution, i.e. the Arrhenius behavior. The difference in η* indicates a different gelation behavior. The reference starch, Starch A, showed a behavior similar to that of Starch B and only the Arrhenius behavior was present. On the other hand Starch C, at high concentrations, showed an increase in originating not only from the Arrhenius behavior but also from the gel network formation. In polymer science, viscoelastic measurements may be used to determine the gel point as the crossover of the viscous and elastic responses (G'=G"), i.e. the phase angle is 45°. When the phase angle falls to a value close to zero, the formation of an elastic network structure is implied [13]. The concentration-dependence of the gel network formation seems to be quite large. At the lower concentrations of Starch B (Fig. 6), the phase angle decreases only slightly. At higher concentrations, the decrease in phase angle becomes more pronounced. The overlapping concentration, c*, was determined to be about 3.5% (w/w) and was lower than the concentration at which the decrease in δ and the increase in |η*| were observed (Fig. 2). The overlap concentration was determined for all molecules in the starch solution, using Starch A. The partic-ulate precipitation and the gel network formed for Starch B respective Starch C may be predominated by the amy-lose fraction. The uniform linear nature of amylose permits intermolecular bonds similar to other linear polymers such as cellulose [1,14]. It is likely that amylose and not amylopectin predominates the formation of the precipitate and the gel network, which may explain the discrepancy between and the onset of rapid changes in 8 and |η*|. At the high concentrations of Starch C, the temperature sweeps were not completely reversible, indicating hysteresis properties of the gel or a long relaxation time compared to the time scale of the experiments. The gelation threshold for the hydrophobically modified starches investigated was found to be different for the different starches. The behavior of the complex viscosity for Starch B was similar to that observed for Starch A after cooling without shear (Fig. 5). However, Starch B showed a substantially higher complex viscosity than Starch A when cooled under shear (Fig. 4). This suggests that the amphiphilic and amphoteric character of Starch B introduced by the quaternary amine group does play an important role in the mechanical behavior of the starch gel. The phase separation and gel formation induced by the interaction of hydrophobic functional groups must not be confused with retrogradation. The starches investigated here showed fully reversible behavior and did not undergo retrogradation during the time-scale of the experiment. 5 Conclusions The hydrophobically modified amphoteric starches showed a temperature-dependent phase behavior; precipitation of a solid-like highly concentrated phase. The oxidized starch showed no phase separation as the temperature was decreased. A zero net charge of the amphoteric starches was shown to promote a reversible precipitation. For the positively charged grade of hydrophobically modified starch, the rheological measurements showed a decrease in the phase angle to below 45°, which indicates the formation of a gel network. The change in phase angle was not as pronounced for the hydrophobically modified starch with zero net charge as it was for the more cationic starch. The temperature-induced changes in rheological properties of the oxidized starch were reversible and the magnitude of the complex viscosity was similar to that of the hydrophobically modified starch of zero net charge. The charge density of the hydrophobically modified amphoteric starch apparently had an influence on whether gelation or precipitation should be the predominating mechanism upon cooling the warm solution to room temperature. Both the gelation and the phase separation behavior were dependent on the polymer concentration. Acknowledgements Lyckeby Stärkelsen, Kristianstad, Sweden is acknowledged for kindly providing starch samples and for valuable discussions. For financial support the Swedish Pulp and Paper Research Foundation, the Knowledge Foundation, and the Swedish Agency for Innovation Systems are gratefully acknowledged. References [1] O. B. Wurzburg, in Modified starches: Properties and uses (Ed. O. B. Wurzburg) CRC Press, Boca Raton, Fl., 1986. [2] D. Glittenberg. A. Becker: Cationic starches for surface sizing. Paper Technol. 1998, 39, 37-41. [3] B. Wesslen: Amphiphilic Graft Copolymers - Preparation and Properties. Macromol. Symp. 1998, 730, 403-410. [4] P. Muller, E. Gruber, C. Brossmer, D. Bischoff: Teilhy-drophobierte kationische Starken fur den Einsatz bei der Oberflachenleimung von Papier. Int. Papierwirtsch. 2000, 2, T22-T28. [5] L M. Landoll: Nonionic Polymer Surfactant. J. Polym. Sci.: Polym. Chem. 1982, 20, 443-455. [6] B. Lindman, A. Carlsson, S. Gerdes, G. Karlstrom, L. Piculell, K. Thalberg, K. Zhang: Polysaccharide-surfactant systems: Interactions, phase diagrams and novel gels, in Food Colloids and Polymers: Stability and Mechanical Properties (Eds. P. Walstra, E. Dickinson), RSC, Cambridge, 1993, pp. 113-125. [7] R. D. Hester, D. R. Squire, Jr.: Rheology of Waterborne Coatings. J. Coat Technol. 1997, 69, 109-114. [8] J. V. Gruber, P. N. Konish: Aqueous viscosity enhancement through helical inclusion complex cross-linking of a hy-drophobically-modified, water-soluble, cationic cellulose ether by amylose. Macromolecules 1997, 30, 5361-5366. [9] L Jarnstrom, P. Hansson, P.-O. Nilsson, C. Brossmer, K. Wiklander: Hydrophobically modified cationic starches for surface treatment. Tappi Coating Conference and Trade Fair, 2000, pp. 99114. [10] A. Jonhed, B. Mesic, C. Hjarthag, L. Jarnstrbm, Starch modifications for surface properties. Pira 3rd Int. Sizing Conf.: Scientific and Technical Advances in Internal and Surface Sizing, Prague, December 2001, Paper 18. [11] L. Jarnstrom, L. Larson, M. Rigdahl, U. Eriksson: Floccula-tion in kaolin suspensions induced by modified starches 2. Oxidised and hydrophobically modified oxidised starch in comparison with poly(vinyl alcohol) and carboxymethylcel-lulose. Colloids and Surfaces A: Physicochemical and Engineering Aspects 1995, 104, 207-216. [12] H.-M. Buchhammer, G. Petzold, K. Lunkwitz: The interaction between oppositely charged polyelectrolytes in the presence of solid surfaces. Colloids and Surfaces A: Physicochemical and Engineering Aspects 1993, 76, 81-85. [13] K. Svegmark, A-K. Hermansson: Shear induced changes in the viscoelastic behavior of heattreated potato starch dispersions. Carbohydr. Polym. 1990, 73, 29-45. [14] R. L. Whistler, J. BeMiller, E. F. Paschall: Starch: Chemistry and Technology, Academic Press, Inc., New York, 1984. (Received: January 27, 2003) (Revised: April 26/June 28, 2003) (Accepted: June 30, 2003)