SCH4U Exam Review May 2013
advertisement
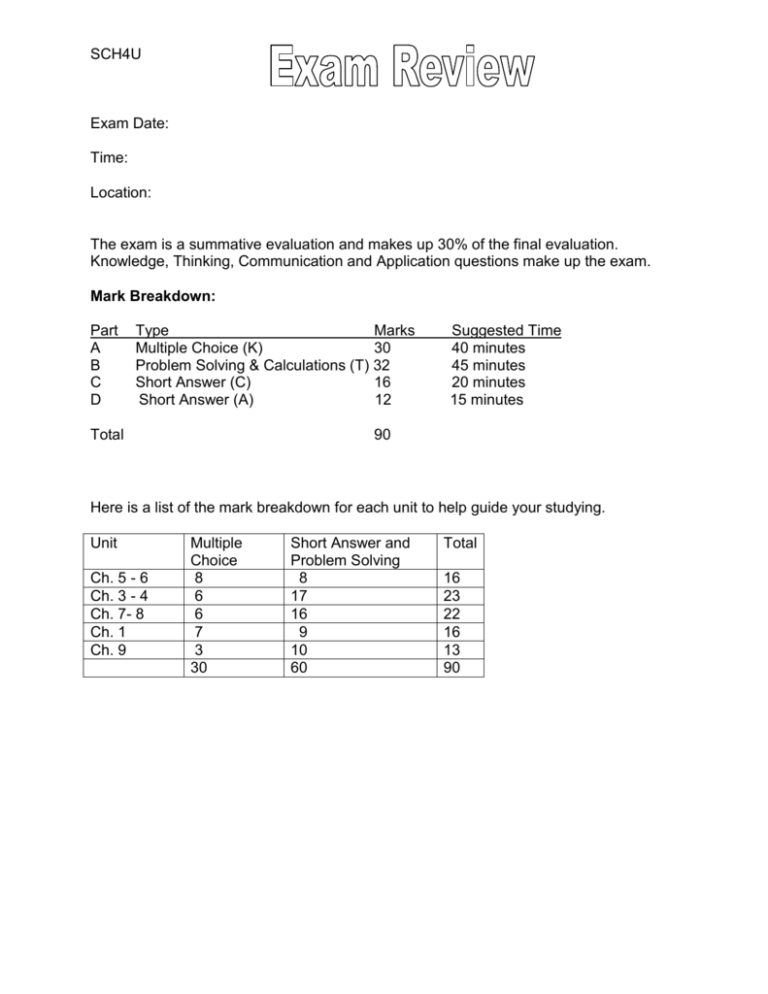
SCH4U Exam Date: Time: Location: The exam is a summative evaluation and makes up 30% of the final evaluation. Knowledge, Thinking, Communication and Application questions make up the exam. Mark Breakdown: Part A B C D Type Marks Multiple Choice (K) 30 Problem Solving & Calculations (T) 32 Short Answer (C) 16 Short Answer (A) 12 Total Suggested Time 40 minutes 45 minutes 20 minutes 15 minutes 90 Here is a list of the mark breakdown for each unit to help guide your studying. Unit Ch. 5 - 6 Ch. 3 - 4 Ch. 7- 8 Ch. 1 Ch. 9 Multiple Choice 8 6 6 7 3 30 Short Answer and Problem Solving 8 17 16 9 10 60 Total 16 23 22 16 13 90 Here are some problems to practice/review to help with the Problem Solving and Short Answer sections of the exam. Unit 1: Energy Changes and Rates of Reaction (Ch. 5 and Ch.6) 5.2 Molar Enthalpy pg. 310 #4,5 5.4 Hess’ Law pg. 329 #4,5 Unit 2: Structure and Properties (Ch. 3 and Ch. 4) 3.5 Quantum Numbers pg. 184 #2,6 3.6 Atomic Structure and Periodic Table pg. 220 #13,15 4.1 Lewis Structure pg. 229 #10 4.2 Valence Bond Theory pg. 235 #11 pg. 238 #20 [Hint: Draw and label orbitals] 4.3 VSEPR Theory pg. 249 #11 4.5 Intermolecular Forces pg. 266 #1 Unit 3: Chemical Systems and Equilibrium (Ch. 7 and Ch. 8) 7.3 Le Chatelier’s Principle pg. 457 #1 pg. 459 #3 7.5 Reaction Quotient pg. 465 #1,2 7.5 Equilibrium Problem pg. 472 #5,6 pg. 476 #7,8 [Hint: Calculation with perfect square or with an imperfect square] 8.7 Acid Base Titration pg. 607 #4-6 [Hint: Weak acid and strong base or strong base with weak acid] Unit 4: Organic Chemistry (Ch. 1) 1.9 Synthesizing Organic Compounds [Hint: An ester or an amide] Unit 5: Electrochemistry (Ch. 9) 9.1 Oxidation and Reduction 9.2 Balancing Redox Reactions pg. 82 #1(a)-(g) pg. 97 #8-10 pg. 662 #18 pg. 668 #2,3 pg. 673 #6,7