PA 81 Electrical conductors and valence electrons
advertisement
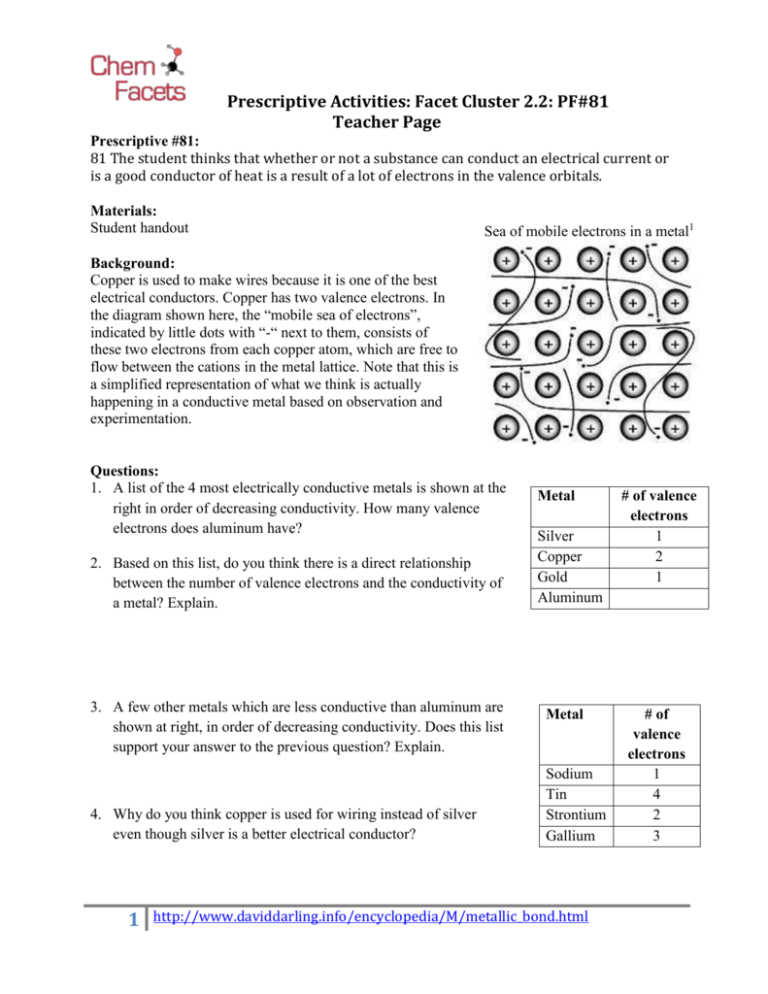
Prescriptive Activities: Facet Cluster 2.2: PF#81 Teacher Page Prescriptive #81: 81 The student thinks that whether or not a substance can conduct an electrical current or is a good conductor of heat is a result of a lot of electrons in the valence orbitals. Materials: Student handout Sea of mobile electrons in a metal1 Background: Copper is used to make wires because it is one of the best electrical conductors. Copper has two valence electrons. In the diagram shown here, the “mobile sea of electrons”, indicated by little dots with “-“ next to them, consists of these two electrons from each copper atom, which are free to flow between the cations in the metal lattice. Note that this is a simplified representation of what we think is actually happening in a conductive metal based on observation and experimentation. Questions: 1. A list of the 4 most electrically conductive metals is shown at the right in order of decreasing conductivity. How many valence electrons does aluminum have? 2. Based on this list, do you think there is a direct relationship between the number of valence electrons and the conductivity of a metal? Explain. 3. A few other metals which are less conductive than aluminum are shown at right, in order of decreasing conductivity. Does this list support your answer to the previous question? Explain. 4. Why do you think copper is used for wiring instead of silver even though silver is a better electrical conductor? 1 Metal Silver Copper Gold Aluminum Metal Sodium Tin Strontium Gallium http://www.daviddarling.info/encyclopedia/M/metallic_bond.html # of valence electrons 1 2 1 # of valence electrons 1 4 2 3







