Grade 5- Physical Science
advertisement

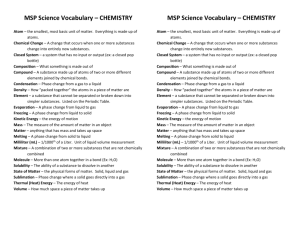
Unit Plan Agenda Science Content Area: Unit Plan Title: Physical Unit Overview of Unit Grade(s) 5 Students will expand their understanding of matter. Through hands on investigations they will measure and calculate mass, volume, and density of substances with appropriate units of metric measurement. Students will understand that all matter is made up of atoms. Atoms of the same substance together create elements. Those elements are organized on the periodic table of elements. Students will observe reactions of substances that produce chemical changes. They will observe the path that visible light takes when it interacts with a prism, mirror, or lens. Essential Question(s) and Enduring Understandings (in grade appropriate terminology). How is matter measured? What is an atom? How is the periodic table of elements organized? What is a chemical reaction & how do you know if you are observing one? What will happen to visible light when it meets with a prism, mirror, or lens? Content Statements and CPI 5.2 Physical Science: Physical science principles, including fundamental ideas about matter, energy, and motion, are powerful conceptual tools for making sense of phenomena in physical, living, and Earth systems science. Strand A. Properties of Matter: All objects and substances in the natural world are composed of matter. Matter has two fundamental properties: matter takes up space, and matter has inertia. Content: The volume of some objects can be determined using liquid (water) displacement. 5.2.6.A.1 Determine the volume of common objects using water displacement methods. Content: All matter is made of atoms. Matter made of only one type of atom is called an element. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements. Content: The density of an object can be determined from its volume and mass. 5.2.6.A.2 Calculate the density of objects or substances after determining volume and mass. Content: All substances are composed of one or more of approximately 100 elements. 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” Content: Pure substances have characteristic intrinsic properties, such as density, solubility, boiling point, and melting point, all of which are independent of the amount of the sample. 5.2.6.A.3 Determine the identity of an unknown substance using data about intrinsic properties. Strand B. Changes in Matter: Substances can undergo physical or chemical changes to form new substances. Each change involves energy. Content: When a new substance is made by combining two or more substances, it has properties that are different from the original substances. 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and react chemically. Strand C. Forms of Energy: Knowing the characteristics of familiar forms of energy, including potential and kinetic energy, is useful in coming to the understanding that, for the most part, the natural world can be explained and is predictable. Content: Light travels in a straight line until it interacts with an object or material. Light can be absorbed, redirected, bounced back, or allowed to pass through. The path of reflected or refracted light can be predicted. 5.2.6.C.1 Predict the path of reflected or refracted light using reflecting and refracting telescopes as examples. Content: Visible light from the Sun is made up of a mixture of all colors of light. To see an object, light emitted or reflected by that object must enter the eye. 5.2.6.C.2 Describe how to prisms can be used to demonstrate that visible light from the Sun is made up of different colors. Student Learning Targets/Objectives: should be connected to content statements. Students will be able to: e.g.: 1- Articulate predictions and imagine ways to test those predictions, using practice gained through class explorations of water quality in the school pond and water supply. Use tools to make accurate measurements and represent measurements by using appropriate metric numbers and units; use measurement data to construct explanations identify varying densities in objects and calculate the density of various household objects understand that all matter is made of atoms understand the basic sub-atomic structure of an atom understand the organization of the periodic table of elements identify chemical reactions observe chemical changes identify properties of visible light illustrate the way visible light reflects and refracts observe how prisms can be used to bend light Strategies/Justifications e.g.: Notebooking will help students clarify their own thinking, identify weaknesses and document progress. Note-booking- students expand thinking and document changing ideas as they make new connections and observations Use scientific process skills to measure mass and volume in the metric system. Highlight balances, scales, and graduated cylinders. Carry out lab activities to calculate density of a substance Identify and locate parts in a model of an atom Participate in activity to organize periodic table of elements Observe chemical changes in a range of different substances Identify by tracing and drawing the paths light takes when it interacts with mirrors and lenses Activities, Lessons, and Assessments Teaching Point #1 Properties of matter- volume, mass Time Frame 1.5 weeks Review What is Matter?- From Lynne Particle movement in states of matter- quick review- Harcourt 444- Lynne has materials Measuring mass and volume of regular shaped objects-Mystery Boxes Harcourt pg 437 Practice reading graduated cylinder (attached) Volume Lesson (attached) Measuring volume of irregular shaped objects- In small groups, plan and conduct an experiment to determine the volume of several small rocks. Using data from their experiments, engage in a guided discussion regarding what volume is and how it can be determined. Volume Displacement Practice (attached) Teaching Point #2 Properties of matter-density Density Column or Dancing Raisins- (http://www.superteacherworksheets.com/scienceprojects/dancing-raisins.pdf) Magic Triangle to calculate density Foldable Density Notes (attached) 1 week Density Investigation (attached) Teaching Point #3 Atoms & subatomic particles 2 weeks Parts of atoms-Atoms Family Album (attached) Atoms Vocab (attached) Atoms Family Math Practice (attached) Intro to Periodic Table Organization Periodic Table of Cookies (http://sciencekit.com/periodic-table-of-cookies-demonstrationteacher-developed-classroom-tested/p/IG0028965/) Element Bingo (Jefferson Lab Element Bingo- Class set of Bingo Boards http://education.jlab.org/beamsactivity/6thgrade/elementbingo/elementbingoclassset.pdf Atomic Models- “Element BINGO Transparency Sheets http://education.jlab.org/nsta/ Periodic Table http://education.jlab.org/itselemental/tableofelements.pdf ) Teaching Point #4 Chemical Changes in Matter 1 week Choose 2 to Demonstrate Chemical Changes Jackie Baggie (on share drive) The Case of Disappearing Grimies (Lynne has) Meat in baggie with peroxide Steel Wool & Penny (attached) Color Change Challenge (attached) Teaching Point #5 Visible Light Visible Light Notes & Intro Diffraction Glasses Activity (attached) Reflection & refraction notebook (powerpoint & notes) Penny & Pencil in Cup (Penny attached) (Lynne has pencil activity) Lightboxes to demonstrate lenses, mirrors, prisms Benchmark Assessment Resources that need to be ordered or located. Technology to be integrated (tools, equipment, software, and online learning) 2.5 weeks