Human Life Cycle 1
advertisement

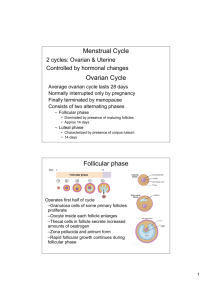
Human Life Cycle 1 - The ovaries, the ovarian cycle and manipulating fertility Anil Chopra 1. Identify the hormones that are important in the maintenance of the different stages of the ovarian cycle. Identify the site of production of these hormones, from the hypothalamus to the ovary. 2. State the stages of follicular development relative to the stages of germ cell (i.e. oocyte) development. 3. Elucidate the development of a follicle from the primordial follicular stage through to the mature follicle. 4. Explain the events which occur at ovulation, relating them to the hormones which are important in bringing about these events. 5. Describe the development of the corpus luteum. 6. Distinguish between the corpus luteum of pregnancy and the corpus luteum of menstruation, indicating the hormones which are important for their maintenance. 7. Define the normal ranges of hormones related to ovarian function. 8. Elucidate the hormonal methods of stimulating the ovary and indicate the site of action of each of these hormones. 9. Describe the ultrasound and biochemical definitions of polycystic ovary syndrome and the methods of ovulation induction which would be appropriate in this condition. 10. Describe the methods of ovulation induction in estrogen deficient women. 11. Describe the complications of ovulation induction treatment. 12. Define the menopause, the symptoms of estrogen deficiency and the possible long term risks associated with estrogen deficiency. 13. Outline the methods of Hormone Replacement Therapy and the clinical indications for additional progesterone therapy. 14. Outline how to monitor the effectiveness of Hormone Replacement Therapy and the necessary screening as part of ongoing treatment. 15. Outline the adverse effects of Hormone Replacement Therapy in both sequential and continuous combined treatments. Two main functions of the ovaries: - production of a mature gametes (ova) by oogenesis - production of steroid hormones (steroidogenesis) The Menstrual Cycle and Fertilisation OVARIAN CYCLE ENDOMETRIAL CYCLE FOLLICULAR PHASE OESTROGEN (17-OESTRADIOL) OVULATION PROLIFERATIVE PHASE LUTEAL PHASE PROGESTERONE and 17-OESTRADIOL SECRETORY PHASE The menstrual cycle is a 28 day cycle that consists of 2 main phases, the follicular and secretory phase. Changes occur due to hormone levels and affect 2 main organs, the ovaries and the endometrium. Endometrial cycle: 1. Menstrual phase – first day of menstruation beginning of cycle, lasts 4-5 days 2. Proliferative phase – post-menstruation build up of endometrium, ~ 9 days. Growth of follicles controlled by oestrogen of follicles. 2-3 fold increase in endometrial thickness, phase of repair and proliferation – epithelium repairs, gland increase number and length, spiral arteries lengthen. Link with ovarian cycle: follicular phase – growth of follicle, when follicle has developed, the ovum is released ovulation (~ day 14) Hormonal control: pituitary – FSH and LH, ovary 3. Secretory phase - ~13 days Progesterone (and oestrogen) Formation of corpus luteum, glandular epithelium, thickening endometrium, spiral arteries. The Ovarian Cycle: 1. Follicular phase At the beginning of the menstrual cycle, ovarian follicles begin to grow and develop Initially, each follicle consists of an outer layer of thecal cells and an inner layer of granulosa cells which surround the ovum. In the maturation process, the ovum increases in size and both groups of cells proliferate under hormonal influence. The thecal cells form two distinct layers: an outer fibrous capsule (the theca externa) and an inner glandular and vascular layer (the theca interna). The development and growth of follicles depends on the synthesis of specific hormone receptors and adequate levels of the relevant hormones; the adenohypophysial gonadotrophins luteinising hormone (LH) and folliclestimulating hormone (FSH). Thecal cells LH receptors granulosa cells FSH and oestrogen receptors. The follicles which have reached this stage of development with the necessary receptors when the gonadotrophins are present in the right quantities ripen. All other follicles will regress and be absorbed into the stroma atresia binding of LH to its thecal cell receptors stimulates these cells to synthesise androgens. FSH binds to the granulosa cells which have a powerful aromatising enzyme (CYP450 aromatase) capable of converting thecal cell androgens to oestrogens. Oestradiol released from the granulosa cells acts directly on these cells further proliferation and growth local positive feedback loop at the cellular level. Insulin-like growth factor, IGF-I, is another hormone which is produced by the growing follicles and it probably plays an important role in growth and development processes. As the plasma oestradiol levels rises during the mid-follicular phase, a selective negative feedback by the oestradiol in the FSH production occurs. This effect, together with inhibin, (produced by the FSH-stimulated granulosa cells), results in a decrease in the plasma concentration of FSH In the maintained basal presence of FSH, together with the increasing quantities of oestrogen, the outer layers of granulosa cells of the remaining (Graafian) follicle now synthesise receptors specific for LH. This Graafian follicle is now capable of further maturation under the influence of its own oestrogens alone, and has no need for further FSH. It enters the final maturation process which consists of the terminal growth of the follicle and the release of its ovum into the peritoneum by a process called ovulation. The stimulus for this event is a sudden surge in LH which is accompanied by a smaller surge in FSH (due to rise in oestrogen levels). Just prior to ovulation, the granulosa cells begin to synthesise progestogens (mainly 17α-hydroxyprogesterone at this stage) instead of converting androgens to oestrogens. These cells also gave reduced capacity to respond to oestrogens and FSH during this short, pre-ovulatory phase. 2. Luteal phase: Following ovulation rise in progesterone production by the outer granulosa cells small increase in the basal body temperature (of the order of 0.3-0.6°C). This is a useful clinical indicator that ovulation has taken place. LH surge causes conversion of the follicular remnants to a corpus luteum Some thecal cells become incorporated with the corpus luteum, composed principally of hyperthyroid granulosa cells. These cells contain high concentrations of lipids and are rich in mitochondria, and their transformation to luteal cells is associated with an increasing production of progesterone as well as oestradiol. If pregnancy occurs, the corpus luteum persists and continues to secrete steroids until the foetoplacental unit can assume this function usually by the twelfth week. If pregnancy does not occur the high plasma concentration of progesterone exerts a powerful negative feedback on the hypothalamo-hypophysial axis, in addition to any negative feedback by oestradiol and other luteal factors such as inhibin. Consequently LH (and FSH) support is withdrawn, the corpus luteum degenerates (luteolysis), and ovarian steroid production decreases. The luteolytic process in humans may also be influenced by the oestrogens produced by the luteal cells themselves. Other luteolytic factors produced by the corpus luteum itself, such as oxytocin, may also be involved in luteolysis. There are a number of changes in the hormonal levels in the blood, the largest of all being the LH surge around day 14, which in turn induces a rise in oestradiol and causes ovulation. ADRENALS AND GONADS ADRENALS ONLY ALDOSTERONE CHOLESTEROL PREGNENOLONE PROGESTERONE 17-OH PROGESTERONE CORTICOSTERONE DEOXYCORTICOSTERONE 11-DEOXYCORTISOL ANDROSTENEDIONE CORTISOL TESTOSTERONE DIHYDROTESTOSTERONE OESTRONE 17- OESTRADIOL MAINLY GONADS NORMALLY The thecal cells of the ovaries produce androgens (along with the adrenal medulla), and the corpus luteum is responsible for the production of 17β-oestradiol and progesterone. Menstrual cycles continue throughout the woman’s life unless fertilisation of the ovum occurs. Hormonal changes in pregnancy In the first 5-6 weeks of pregnancy, the maternal ovaries produce the main gonadal steroids. The rising levels of oestrogens and progesterone cause a fall in LH and FSH levels. Slowly, the developing foetoplacental unit begins to form more and more hormones namely human Chorionic Gonadotrophin. This maintains the corpus luteum throughout pregnancy (normally it is broken down). Ovarian Function, Hyperstimulation and Management Anovulation: the absence of ovulation due to abnormal endocrinology. It is a common cause of infertility, normally easily treated. Presentation • Amenorrhoea (>6 months) – no period in the last 16 months • Oligomenorrhoea (cycle >42 days) – irregular periods • Irregular menses (e.g. cycles varying between 2 and 6 weeks in duration) Causes of Amenorrhoea Primary ovarian failure Hypothalamic/pituitary o Hyperprolactinaemia o Weight loss/exercise related o Idiopathic (isolated GnRH deficiency) Polycystic ovary syndrome Genital tract disorder Pregnancy Causes of Oligomenorrhoea Polycystic ovary syndrome Perimenopausal Recovered weight loss Uncertain cause Polycystic Ovary Syndrome • • • • • • Commonest cause of anovulation (~80% cases of anovulatory infertility) Characterised by androgen excess clinical (hirsutism/acne/frontal hair loss) and/or biochemical (raised testosterone) Mechanism of anovulation uncertain: arrested antral follicular development Anovulatory menses or oestrogen-replete amenorrhoea (have enough oestrogen) Clinical and/or biochemical evidence of androgen excess Polycystic ovaries Polycystic Ovaries on Ultrasound scan normal normal PCO PCO more follicles, more stroma Endocrine Diseases Associated with PCOS • Hypothalamic-pituitary disease • prolactinoma • acromegaly • Cushing’s • non-functioning tumours • Sheehan’s syndrome • Adrenal disease • CAH, - enzyme block of cortisol production • virilising tumours (androgen secreting tumours) • Addison’s disease • Thyroid disease • Hypothyroidism • hyperthyroidism Investigations for Anovulation FSH, LH (3-11 u/l) Prolactin (50 - 500 mu/l) Assessment of oestrogen status (if amenorrhoea) o Serum oestradiol (100-1200 pmol/l) o early follicular phase E2 (100-400 pmol/l) o Endometrial thickness o Progestogen withdrawal test (medroxyprogesterone 5mg/day for 5 days). o TSH (0.5-5.0 mu/l) o Pelvic ultrasonography o Testosterone (0.5-3.0 nmol/l)# o Progesterone (>20 nmol/l) 3 Different Outcome Groups • High FSH, low E2 = primary ovarian failure • Normal/low FSH, low E2 = hypothalamic/pituitary disorder – Measure prolactin • Ultrasound scan with normal FSH, normal E2 (± high LH) = PCOS normal PCOS probably related to abnormal endocrine environment • leading to relative deficiency of FSH Treatment Treat the underlying cause e.g. if they are not ovulating due to weight loss, then treat the weight loss. If Cushing’s syndrome, treat pituitary adenoma. Induction of Ovulation • Antioestrogens (clomiphene) (PCOS) • Gonadotrophins (hypothalamic/pituitary causes; PCOS) • Pulsatile GnRH (hypothalamic amenorrhoea) • Dopamine agonists (hyperprolactinaemia) Superovulation: to override the physiology and stimulate the development of many follicles e.g. for IVF treatment. GnRH treatment is generally effective in treatment of anovulation and PCOS. The first choice of treatment is Clomiphene. This is a selective oestrogen receptor modulation SERM which works by blockade of the oestrogen receptors in the hypothalamus and thus increasing the release of GnRH. If the treatment with Clomiphene fails to work then administer human menopausal Gonadotrophin HMG. The Gonadotrophins LH and FSH were purified from urine and injected. Now they are available as recombinant hormones. The outcome of the treatment is affected by any pre-treatment, any other initial causes of anovulation, and BMI (BMI of over 25 can increase the threshold of FSH and reduce pregnancy rate). Weight loss dramatically increases rate of ovulation. If neither of these treatments works then one can perform a Laporoscopic ovarian diathermy. This is a surgical procedure where multiple diathermy points are introduced (holes) to each ovary. The trauma to the cortex of the ovary makes the ovaries more responsive to endogenous gonadotrophin secretion. The risks include the induction of multiple pregnancies and ovarian hyperstimulation (Symptoms include abdominal pain, bloating with nausea and constipation, and if sever requires intensive care). Menopause and Hormone Replacement Therapy In the last 100 years the average life expectancy has risen by around 30 years, which means that women are living longer past the age of menopause. The menopause normally occurs between the ages of 45-55 preceded by a “perimenopause” – a period of oligomenorrhoea. Often women are infertile before they get to the menopause. Females are born with a life-time’s supply of oocytes. Only one oocyte per month matures into the ovum. The rest undergo a process of atresia. These germ cells are not replenished and hence the number and quality of oocytes declines with age along with frequency of intercourse. Ageing LH remains stable throughout life The FSH levels increase in the early follicular phase from the ages of 35-40 onward. This is possibly due to the: o Decreased inhibin B secretion from antral follicles o Increased FSH may explain increase in dizygotic twins Cycle lengths get shorter with age due to shorter follicular phase Decreased number of antral follicles with ageing. Variable cycles (reduced availability of small antral follicles for selection?). Peri-Menopause This is often a time of infertility and oligomenorrhoea. As ovarian reserve of follicles declines, oestradiol levels fall and FSH increases. The high levels of FSH may “hyperstimulate” the next “crop” of follicles (Multiple antral follicles and/or follicular cysts may develop) Oestradiol levels may transiently become supraphysiological and FSH is suppressed and as a result, cause abnormal menstrual cycles. Post-Menopause • Androgen production decreases but still produced by adrenal and, in small amounts, by ovary • Low levels of oestrogen in circulation produced mainly by peripheral conversion of androgen • Gonadotrophins remain high Diagnosis of Menopause In order to diagnose menopause, patient must have not had a menstrual cycle in 12 months, a rise in LH and FSH levels to over 20u/l, and a fall in oestradiol levels. The lack of oestrogen has a number of effects: Vasomotor • Hot flushes/sweating • Palpitations • Headaches Genitourinary tract • Vaginal dryness and dyspareunia • Urge incontinence, dysuria, urinary frequency Neurological/ Psychological • Forgetfulness, lack of concentration, • depression, irritability, anxiety • loss of libido • tiredness, insomnia Skeletal • Bone loss leading to osteopoenia/osteoporosis Cardiovascular • Increased risk of CHD and stroke? • Risk of cardiovascular events lower in premenopausal female population compared to male population • Post menopause rates of cardiovascular disease increase and eventually equal those in the male population Hormone Replacement Therapy Hormone replacement therapy aims to treat the symptoms of oestrogen deficiency and prevent osteoporosis and cardiovascular risk. Treatment is started as soon as the onset of menopausal symptoms. There are 3 main types of HRT: - Oestrogen alone - Combined oestrogen and progestogen - SERM – selective oestrogen receptor modulator The side effects are predominantly oestrogenic and include breast enlargement, nausea, vomiting, mood changes, migraine and headache. Its long term side effects include: • Small but significant increase in risk of breast cancer, but the risk is related to duration of HRT use, not age at which treatment is started. Foetal Growth Rates Human growth differs in rate and occurs up until around the age of 18 in boys and 16 in girls. Growth is fastest at birth and gradually declines with a pronounced increase during the adolescent growth spurt. The foetus grows in a distinctive way, with the different organs growing at different speeds. There are a number of different environmental factors that affect foetal growth including the maternal age, weight, nutrition, and drug use (smoking, alcohol). It can also be affected by foetal genotype, gender and hormones.