Day 03- Lab Galvanic Cell revised F14
advertisement
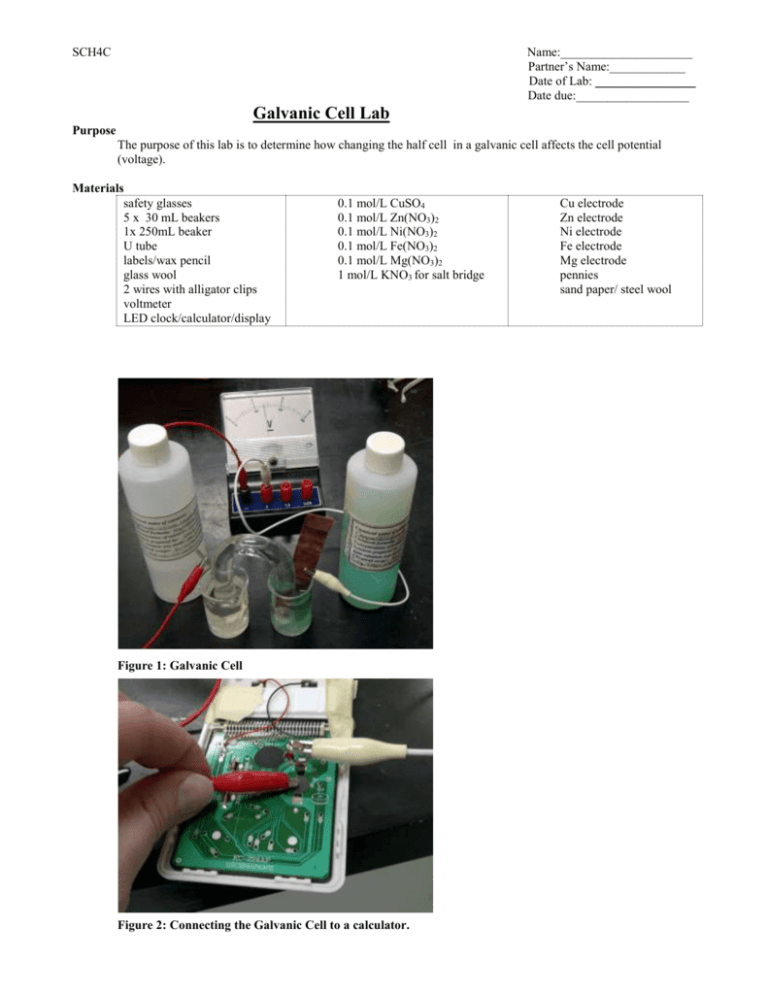
SCH4C Name:_____________________ Partner’s Name:____________ Date of Lab: ________________ Date due:__________________ Galvanic Cell Lab Purpose The purpose of this lab is to determine how changing the half cell in a galvanic cell affects the cell potential (voltage). Materials safety glasses 5 x 30 mL beakers 1x 250mL beaker U tube labels/wax pencil glass wool 2 wires with alligator clips voltmeter LED clock/calculator/display 0.1 mol/L CuSO4 0.1 mol/L Zn(NO3)2 0.1 mol/L Ni(NO3)2 0.1 mol/L Fe(NO3)2 0.1 mol/L Mg(NO3)2 1 mol/L KNO3 for salt bridge Figure 1: Galvanic Cell Figure 2: Connecting the Galvanic Cell to a calculator. Cu electrode Zn electrode Ni electrode Fe electrode Mg electrode pennies sand paper/ steel wool SCH4C Name:_____________________ Partner’s Name:____________ Date of Lab: ________________ Date due:__________________ Procedure Build the Galvanic Cells and Determine Cell Potentials 1. Sand the surfaces on one end of each electrode until it is shiny. 2. Fill each 30mL beaker with a different metal ion solution and label them. 3. Fill U tube salt bridge with 1 mol/L KNO3 and stuff some glass wool in each end. 4. Set up your each cell as shown in the picture. 5. If the needle on your voltmeter swings the wrong way, switch the way the wires are attached. 6. Record the cell potential in Table 1. 7. Try to power a clock or calculator with a LCD screen by removing the battery and connecting it to your cell. 8. Record your observations in Table 1. 9. Repeat for the other cells. 10. Take the electrodes out of solution, rinse them with water and dry them with a paper towel. Do any of the electrodes look different where they were submerged in solution? Record your observations in Table 2. Observations Table 1: Voltages and LCD test results for Galvanic Cells (6 marks, C) Cell 1 2 3 4 5 6 Cell Potential (V) Did the Clock/Calculator Work With this Cell? (Y/N) Cu/Zn Cu/Ni Cu/Fe Cu/Mg Mg/Zn Mg/Fe NOTE: In this situation, iron is more likely to form Fe +2(aq) than Fe+3(aq) Table 2: Physical description of the electrode after being connected to the galvanic cell Electrode Dry Electrode After Reaction Cu Zn Ni Fe Mg SCH4C Name:_____________________ Partner’s Name:____________ Date of Lab: ________________ Date due:__________________ Questions 1. Will the cells that contain copper work if you use a penny instead of a Cu electrode? Try it! 2. What happens if you remove the voltmeter? 3. What happens if you remove the salt bridge? 4. a) In a galvanic cell, the metal higher on the activity series is always oxidized and the metal lower on the activity series is reduced. Oxidation occurs at the anode and reduction occurs at the cathode. Using this information, identify the anode and the cathode for each cell in Table 1. (6 marks, I) b) Which metal was the anode of the cell most often? (1 mark, I) c) Which metal was the cathode of the cell most often? (1 mark, I) d) Which cell had the largest cell potential (voltage)? (1 mark, I) e) Which cell had the smallest cell potential? (1 mark,I) e) Cu is lowest on the activity series. For the cells with Cu and another metal, what happed to the cell potential as the reactivity of the other metal increased (higher on activity series)? (2 marks, I) f) Mg is highest on the activity series. For the cells with Mg and another metal, what happened the cell potential as the reactivity of the other metal increased (higher on activity series)? (2 marks,I) g) What happened when you tried to use the penny as an electrode? Was the voltage the same as with the Cu strip? ( 1 mark, C) 5. For the galvanic cell with the highest voltage: a) Write the anode, cathode, and overall cell reactions that occur. (4 marks, I) SCH4C Name:_____________________ Partner’s Name:____________ Date of Lab: ________________ Date due:__________________ b) c) d) e) Draw a LARGE diagram of your galvanic cell (2 marks,C) Label the two electrodes, two solutions, anode, cathode, voltmeter and salt bridge (4 marks, C) Show where reduction and oxidation occur ( 1mark,C) Show the direction of electron flow in the wire and the direction of ion flow. (1 mark,C) f) Predict which electrode increases in mass and which electrode decreases in mass. Justify your predictions. (3 marks, I) g) Use your solubility table and explain why KNO3 makes a better a salt bridge for this lab instead of K3PO4. (3 marks, C) 6. About how much voltage does it take to power the LCD? Explain how you know. (2 mark, I)