Relationships Among Elements
advertisement

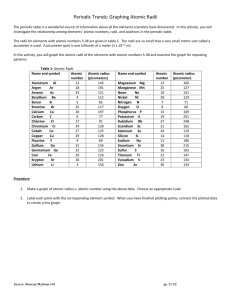
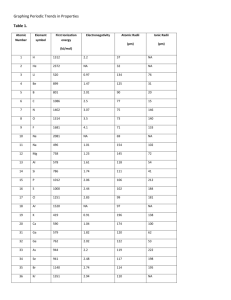
Periodic Trends: Graphing Atomic Radii - KEY Questions and Conclusions: 1. Look at the shape of your graph. What patterns do you observe? The graph peaks, falls into valleys, and rises again with a little peak appearing just before each of the high peaks. 2. What family is represented by the high peaks on your graph? Alkali metals (Group 1) 3. What family is represented by the low peaks on your graph? Halogens (Group 17) 4. What family is represented by the smaller peaks just before the high peaks in your graph? Noble gases (Group 18) 5. What do you notice about the radii of the elements at the high peaks as you move from left to right on your graph? Look at your periodic table and find the element that represents each high peak. Where does each high peak begin in the periodic table? The radii increase. Each peak represents a group. 6. What happens to the radii of the elements between two highest peaks? What does each of these groups of elements represent? The radii become smaller. Each group of elements represents a period. 7. How can graphs such as the one you made help to predict the properties of elements that have not been discovered yet? A graph of discovered elements predicts the atomic radii of any undiscovered elements that have atomic numbers within the range of the discovered elements. Undiscovered elements would be expected to have properties similar to discovered elements that occupy comparable positions on the graph. 8. How do the radii of metals in each period compare with the radii of nonmetals in that period? Metals have larger radii than the nonmetals in the same period. Source: Glencoe/McGraw-Hill pp. 21-22