Chemical Safety Appendix 6

SMILE
Johns Hopkins University
Baltimore, MD USA
Appendix 6 – Chemical Safety SOP
Jo Shim MBA, MT (ASCP)
Document Number Effective Date
Author(s), Name &
Title
International QA/QC Coordinator
Fac10-13-SOP
Appendix 6
17 Feb 2009
SMILE Comments: This document is provided as an example only. It must be revised to accurately reflect your lab’s specific processes and/or specific protocol requirements. Users are directed to countercheck facts when considering their use in other applications. If you have any questions contact SMILE.
Approved By
Name, Title Signature Date
SOP Annual
Review
Name, Title Signature Date
Revision
History
Version # [0.0]
2.0
Distributed
Copies to
Name (or location)
Revision Date
[dd/mm/yy]
17 Feb 2009
Description (notes)
Reformatted to meet SMILE Resource Template format requirements.
# of copies Name (or location) # of copies
Fac1.0-13 Appendix 6-Chemical Safety Version#: 2.0
Page 1 of 12
SMILE
Johns Hopkins University
Baltimore, MD USA
I acknowledge that I have read, understand and agree to follow this SOP.
Name (print) Signature
Fac1.0-13 Appendix 6-Chemical Safety Version#: 2.0
Page 2 of 12
Date
SMILE
Johns Hopkins University
Baltimore, MD USA
APPENDIX 6 – Chemical Safety
CHEMICAL HAZARDS
I. DEFINITIONS
1.
2.
Hazardous material or chemical - any chemical, which is a physical or health hazard
MSDS – Material Safety Data Sheet
II. TABLE OF CONTENTS
1. Introduction
2. Chemical hazards in the laboratory: OSHA'S "Right to Know" law
3. Classification
4. Labeling
5. Chemical Lists
6. Storage of corrosives
7. Storage of flammables
8. Handling caustic materials
9. Breaks and spills
10. Mercury
11. Disposal of chemical wastes
12. Carcinogens
13. Suspected carcinogens
INTRODUCTION: III.
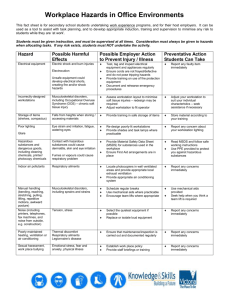
A number of routine procedures in a clinical laboratory involve the use of highly caustic, poisonous, or flammable reagents. These should be appropriately labeled to indicate the hazards. Read labels and observe precautions.
Failure to follow safe practices is cause for disciplinary action.
IV. CHEMICAL HAZARDS IN THE LABORATORY: OSHA'S "RIGHT TO KNOW" LAW
1. The Occupational Safety and Health Administration (OSHA) has issued regulations regarding education of employees regarding hazardous chemicals present in the workplace. All laboratories, including clinical laboratories, will be required to:
Have Material Safety Data Sheets (MSDS) accessible to employees for chemicals used in the laboratory. An MSDS is a printed sheet (or computer file) listing product identification, precautionary labeling, hazardous components, fire and explosion data, health hazard data, spill and disposal procedures and similar information on individual chemicals or mixtures.
MSDS ’s can be requested from the QA or Lab Manager, the manufacturer or obtained online at http://www2.siri.org/msds/index.php.
Label containers of chemicals properly; manufacturer's labels are acceptable.
Train employees to recognize potential hazards in the workplace and proper procedures for handling hazardous substances.
Prepare a list of hazardous chemicals used in lab for inventory. The list of hazardous chemicals used in the laboratory is to be updated and reviewed annually.
2. The employee's responsibility regarding chemical hazards.
Fac1.0-13 Appendix 6-Chemical Safety Version#: 2.0
Page 3 of 12
SMILE
Johns Hopkins University
Baltimore, MD USA
V.
Know the chemical hazards of the reagents you work with. Consult the procedure manuals and refer to the MSDS files to learn the hazards of any chemical that you use before you start a job. Not all prepackaged mixtures have an MSDS. Look at the MSDS of key components.
Handle and dispose of chemicals using good laboratory practice and as described in the procedure manuals.
Use safety appliances and PPE such as lab coat, gloves, goggles and fume hoods as appropriate. Refer to MSDS file where appropriate. Notify a supervisor if any discrepancy exists.
Consult your supervisor if you have concerns regarding the hazard of any chemical or procedure.
3. The Employee's Rights regarding Chemical Hazards.
See the Chemical Information List and MSDS for hazardous substances in your workplace within one day of your request.
Be provided with one copy of the list of substances you use and the corresponding MSDS (or the means to make a copy at no cost) within five days of a request.
Be trained on the hazards of the chemicals in your workplace, on the appropriate equipment and methods necessary to protect you from the hazards, and on associated emergency procedures.
Refuse to work with a hazardous chemical if denied access to information about that chemical.
CLASSIFICATION - Dangerous chemicals are classified as follows:
1.
2.
3.
Caustic or corrosive: Acids and alkalis may cause burns of skin, mouth, or eyes and may also cause damage to equipment and storage areas.
Poisons: Almost any substance in quantity can be poisonous. For these purposes, a poison will be classified as a substance which may cause death or serious effects if relatively small amounts are inhaled, ingested, or contact the skin (such as concentrated phenols). Poisons may be gas, liquid, or solid
Carcinogens: Substances designated by OSHA as carcinogenic (cancer causing) require special handling.
4. Flammables: Such materials that easily ignite/burn and serve as fuel for a fire.
5. Explosive: Materials which may explode under special circumstances.
VI. LABELING:
1. Manufacturers are required to disclose and display appropriate hazard warnings on all chemicals however, regular periodic inventories may reveal containers purchased before manufacturers were required to use adequate and precautionary labeling. Therefore, the laboratory is also required to ensure that containers of hazardous chemicals in use or in storage are labeled with identity or contents of the container and the applicable hazard warnings.
Fac1.0-13 Appendix 6-Chemical Safety Version#: 2.0
Page 4 of 12
2.
3.
4.
5.
SMILE
Johns Hopkins University
Baltimore, MD USA
Existing labels on containers carrying hazardous chemicals should not be removed or defaced unless the container is immediately marked with the required re-labeling information.
Any secondary container into which hazardous chemicals are transferred from originally labeled containers must also be labeled with:
The chemical identity of the contents
Precautionary handling hazards.
Date of receipt
Date of preparation and/or date placed in service,
Dilution ratio, if applicable
Hazardous characteristics, i.e., caustic, corrosive, poisonous, carcinogenic, etc.
Date of expiration.
Labels or other forms of warning must be legible, in the same language as that used by laboratory personnel and prominently displayed on the container.
The only permissible exceptions to this requirement are containers intended for immediate use only by the person who does the transfer and only within the work shift in which the transfer was made. Unlabeled containers of chemicals should not be used; such materials should be disposed of promptly.
Certain manufacturers use the National Fire Protection Association System of identification. The
National Fire Protection Association (NFPA 704) "Identification of the Hazards of Materials" is a symbol system. The diamond identifies the health, flammability, and reactivity hazards as well as the severity using a 0-4 gradient, with 4 as the highest hazard. This system was designed to be easily understood and adequate for fire fighters to evaluate hazards in emergencies at the expense of some specificity and comprehensiveness.
6. The five degrees of hazard have these meanings to fire fighters:
4 - Too dangerous to approach with standard fire-fighting equipment and procedures.
Withdraw and obtain expert advice on how to handle.
3 - Fire can be fought using methods intended for extremely hazardous situations, such as unmanned monitors or personal protective equipment which prevents all bodily contact.
2 - Can be fought with standard procedures, but hazards are present which require certain equipment or procedures to handle safety.
1 - Nuisance hazards present which require some care, but standard firefighting procedures can be used.
0 - No special hazards and no special measures.
7. Health Hazards (BLUE)
4 - Materials too dangerous to health to expose fire fighters. A few whiffs of the vapor could cause death. Protective clothing and breathing apparatus, available to the average fire department personnel, will not provide adequate protection against inhalation or skin contact
Fac1.0-13 Appendix 6-Chemical Safety Version#: 2.0
Page 5 of 12
SMILE
Johns Hopkins University
Baltimore, MD USA
8. with these materials.
3 - Materials extremely hazardous to health but areas may be entered with extreme care.
2 - Materials hazardous to health but areas may be entered freely with self-contained breathing apparatus.
1 - Materials only slightly hazardous to health.
0 - Materials which on exposure under fire conditions, should offer no health hazard beyond that of ordinary combustible material.
Flammability Hazards (RED)
4 - Very flammable gases or very volatile flammable liquids.
3 - Materials that can be ignited under almost all normal temperature conditions. Water may be ineffective because of the low flash point in the materials.
2 - Materials that must be moderately heated before ignition will occur. Water spray may be used to extinguish the fire because the material can be cooled below its flash point.
1 - Materials that must be preheated before ignition can occur. Water may cause frothing if it gets below the surface of the liquid and turns to steam. However, water fog gently applied to the surface will cause a frothing which will extinguish the fire.
0 - Materials that will not burn.
Reactivity or Stability Hazards (Yellow) 9.
4 - Materials which are readily capable of detonation at normal temperatures and pressures. If they are involved in a massive fire, vacate the area.
3 - Materials which, when heated and under confinement, are capable of detonation and that may react violently with water. Fire fighting should be conducted from behind explosionresistant locations.
2 - Materials which will undergo a violent chemical change at elevated temperatures and pressures but do not detonate.
1 - Materials which are normally stable but may become unstable in combination with other materials or at elevated temperatures and pressures. Use normal precautions as in approaching any fire.
0 - Materials which are normally stable and, therefore, do not produce any reactive hazard to fire fighters.
Special Hazards (White)
OX
ACID
ALK
COR
Denotes an oxidizer, a chemical which can greatly increase the rate of combustion/fire.
Unusual reactivity with water. This indicates a potential hazard using water to fight a fire involving this material.
Indicates that the material is an acid or corrosive material with a pH less than 7.0
Denotes an alkaline material (base) or caustic material with a pH greater than 7.0
Denotes a material that is corrosive (can be an acid or a base).
This is another symbol used for corrosive.
Used to denote a poison or highly toxic material. See also: CHIP Danger symbols.
Fac1.0-13 Appendix 6-Chemical Safety
Denotes radioactive hazards. Extremely hazardous when inhaled.
Version#: 2.0
Page 6 of 12
SMILE
Johns Hopkins University
Baltimore, MD USA
Indicates an explosive material. Easily recognized by their instability rating.
VII. INCOMPATIBLE CHEMICALS
Chemical Incompatible with:
Alkaline and alkaline earth metals, such as sodium, potassium, cesium, lithium, magnesium, calcium, aluminum
Acetic acid
Acetone
Acetylene
Ammonia (anhydrous)
Ammonium nitrate
Carbon dioxide, carbon tetra-chloride, and other chlorinated hydrocarbons, any free acid or halogen. Do not use water, foam, or dry chemical on fires involving these metals.
Chromic acid, nitric acid, hydroxyl-containing compounds, ethylene glycol, perchloric acid, peroxide, and permanganates
Concentrated nitric and sulfuric acid mixtures
Chlorine, bromine, copper, silver, fluorine, and mercury
Mercury, chlorine, calcium hypochlorite, iodine, bromine, and hydrogen fluoride
Acids, metal powders, flammable liquids, chlorates, nitrates, sulfur, finely divided organics or combustibles
Aniline
Bromine
Calcium carbide
Calcium oxide
Carbon, oxide
Copper
Chromic acid
Chlorine
Chlorine dioxide
Fluorine
Hydrocyanic acid
Hydrogen peroxide
Nitric acid, hydrogen peroxide
Ammonia, acetylene, butadiene, butane and other petroleum gases, sodium carbide, turpentine, benzene, and finely divided metals
Water (see also acetylene)
Water
Calcium hypochlorite
Acetylene, hydrogen peroxide
Acetic acid, naphthalene, camphor, glycerine, turpentine, alcohol, and other flammable liquids, paper, or cellulose
Ammonia, acetylene, butadiene, butane and other petroleum gases, hydrogen, sodium carbide, turpentine, benzene, and finely divided metals
Ammonia, methane, phosphine, and hydrogen sulfide
Isolate from everything
Nitric acid, alkalis
Copper, chromium, iron, most metals or their salts, any flammable liquid, combustible materials, aniline, nitromethane
Ammonia, aqueous or anhydrous Hydrofluoric acid, anhydrous
(hydrogen fluoride)
Hydrogen sulfide
Hydrocarbons (benzene, butane, propane, gasoline, turpentine)
Iodine
Mercury
Nitric acid (concentrated)
Fac1.0-13 Appendix 6-Chemical Safety
Fuming nitric acid, oxidizing gases
Fluroine, chlorine, bromine, chromic acid, sodium peroxide
Acetylene, ammonia (anhydrous or aqueous)
Acetylene, fluminic acid ammonia
Acetic acid, aniline, chromic acid, hydrocyanic acid, hydrogen sulfide, flammable liquids, flammable gases, and nitritable substances
Version#: 2.0
Page 7 of 12
SMILE
Johns Hopkins University
Baltimore, MD USA
Chemical
Nitroparaffins
Oxygen
Inorganic bases
Incompatible with:
Oils, grease, hydrogen, flammable liquids, solids, or gases
Oxalic acid
Perchloric acid oils, organic amines or antioxidants
Peroxides, organic
Phosparus (white)
Potassium chlorate
Potassium perchlorates
Potassium permanganate
Silver compounds
Sodium
Sodium nitrate
Sodium oxide
Sodium peroxide
Sulfuric acid
Silver, mercury
Acetic anhydride, bismuth and its alloys, alcohol, paper, wood, grease,
Acids (organic or mineral); avoid friction
Air, oxygen
Acids (see also chlorate)
Acids (see also perchloric acid)
Glycerine, ethylene glycol, benzaldehyde, any free acid
Acetylene, oxalic acid, tartaric acid, fulminic acid, ammonium
See alkaline metals (above)
Ammonium nitrate and other ammonium salts
Water, any free acid
Any oxidizable substance, such as ethanol, methanol, glacial acetic acid, acetic anhydride, benzaldehyde, carbon disulfide, glycerine, ethylene glycol, ethyl acetate, methyl acetate, and furfurol
Chlorates, perchlorates, permanganates
Zirconium Prohibit water, carbon tetrachloride, foam, and dry chemical or zirconium fires
VIII. STORAGE OF CORROSIVES:
1. Store caustic and corrosive materials near the floor to minimize danger of bottles falling from shelves.
2. Separate containers to facilitate handling.
3. Store organic acids (acetic acid and acetic anhydride) separately from strong oxidizing agents
(sulfuric, nitric, or perchlorate) to prevent interaction of fumes and corrosion of storage cabinets.
4. Bottle carriers must be used for containers of acid over 500 mL in size.
IX. STORAGE OF FLAMMABLES:
1. An approved flammable storage cabinet is required. Do not store more than 37 liters of flammable liquid in an individual fire area. Not more than 227 liters are allowed in a flammable storage cabinet unless approved by [ Health Safety Officer or Lab Manager ].
2. Quantities of 3.5 liters or larger must be stored in approved flammable material storage cabinets.
If a reagent must be stored in glass for purity, the glass container may be placed in a bottle to lessen the danger of breakage.
3. Small quantities (working amounts) may be stored on open shelves, but bulk storage (more than
18 liters) must be in a flammable liquid storage room.
4.
5.
Do not store flammables in areas exposed to direct sunlight.
Ether is a particular hazard; only small containers (one pint or less) should be used. Once
Fac1.0-13 Appendix 6-Chemical Safety Version#: 2.0
Page 8 of 12
SMILE
Johns Hopkins University
Baltimore, MD USA opened, containers must be stored in an explosion-proof enclosure (preferably a vented flammable storage cabinet).
6.
7.
Storage of flammables in refrigerators shall be in approved flammable material refrigerators only.
Small amounts of residual ether may be disposed of by leaving the open container in an explosion-proof fume hood.
X. HANDLING CAUSTIC MATERIALS:
1.
2.
3.
4.
If large quantities of acids or alkalis are being used, use a shield or barrier or work in a sink or fume hood so breaks or spills can be controlled.
Wear aprons, gloves, and eye protection devices when handling highly corrosive materials.
Do not pipette by mouth.
Do not sniff reagents.
XI. DILUTION:
1.
2.
3.
4.
Use great care and add reagents SLOWLY.
Always add acid to water, NEVER water to acid.
Allow acid to run down the side of the container and mix slowly by gentle rotation.
Avoid overheating.
XII. BREAKS AND SPILLS:
1.
2.
Skin/eye/mouth contact: wash area immediately & seek medical attention.
Clothing spills: take item of clothing off immediately to avoid soaking through to skin. This includes belts and shoes (if affected). Rinse the affected area in the safety shower and seek medical attention.
3. Refer to the MSDS to determine appropriate clean-up procedures using the following information:
Type of material
Identification - common or chemical name
Volume of spill
Degree of danger to others and property
4. Contain spills to prevent the spread of spilled material using any action designed for this purpose. Evacuate area if irritating odors or dangerous vapors exist.
5. Clean up spill with sand or absorbent materials if it consists of acid, base or flammables. Wash area thoroughly after clean up.
6. Toxic or explosive material spills shall be handled by [ indicate personnel/department ]. Notify them of the spill at [ number ] and evacuate the area.
7. Large flammable spills, beyond the ability to handle safely, shall be handled by [ indicate personnel/department ]. Notify them of the spill at [ number ] and evacuate the area.
8.
9.
Small quantities of miscible liquids may be flushed down the sink with copious amounts of water.
If exposure to a hazardous chemical has occurred, the employee shall report promptly to the
Occupational Injury Clinic or to the Adult Emergency Room when the clinic is closed. A Report of
Incident form is to be complet ed by the individual’s supervisor – see appendix 2.1.
Fac1.0-13 Appendix 6-Chemical Safety Version#: 2.0
Page 9 of 12
SMILE
Johns Hopkins University
Baltimore, MD USA
XIII. MERCURY:
1. Mercury Spills –
2.
Minimize mercury spills by using appropriate substitutes whenever possible.
Evacuate the spill area. Ensure than none of the evacuated personnel has mercury contaminated clothing
Turn down the temperature. The cooler the temperature, the less mercury will vapors will be released in the air. Vapors are colorless and odorless. However, if the ventilation or air conditioning system vents to other areas, it must be turned off!
Close interior doors.
Report the spill if required by local regulations
Spills less than 3 mL ’s
Contain spills by surrounding contaminated area with wet paper towels.
Surround or block off the mercury to keep it from spreading onto sloped or porous surfaces. Divert all mercury away from floor drains, cracks, or crevices that may impact groundwater, surface water, and soils.
Wear appropriate PPE: lab coat, gloves, goggles & mask (gas-mask, if available). Remove all jewelry to prevent amalgamation.
Assemble cleaning supplies:
Required a. Eye dropper or disposable pipette h. b. Flashlight c. Plastic container with lid d. Bio-hazard bag with re-sealable closure e. Paper-towels f. Rubber Squeegee g. Plastic dust pan or rigid paper (index card) k. l. i. j.
Optional
Sulfur – Yellow powder that forms mercuric sulfide upon contact and turns brown.
Powdered Zinc – amalgamates with mercury to form a solid.
Sodium Sulfide Solution
Acetic Acid
Hydrogen Peroxide
Do not use a broom to pool droplets as this creates dust and smaller particles.
Using the eyedropper or disposable pipette, pick up all visual mercury droplets.
With the aid of a flashlight or other high intensity light, clean-up any remaining mercury with the paper-towel or squeegee and dust-pan.
If available, dust the area with sulfur or zinc powder to identify and clean any remaining mercury.
The presence of scattered mercury droplets may also be detected by a sodium sulfide solution. This solution may be sprayed on an affected person (but NOT the eyes, mucous membranes, or the mouth). Any mercury present will show up as dark, reddish brown stains.
Residual mercury may then be uplifted by wiping the area with a acetic acid-soaked swab, followed by a peroxide wipe.
Place all mercury contaminated items in a primary plastic container. Seal closed. Place the primary plastic container into a secondary biohazard bag and seal closed. All materials that come in contact with the mercury must be disposed of in this fashion.
Mark the hazardous waste as: Elemental Mercury – Hazardous Waste.
Do dispose of in accordance with local safety regulations. Contact [ indicate personnel/department ] for disposal.
3. Large Mercury Spills - Mercury spills shall be handled by [ indicate personnel/department ]. Notify
Fac1.0-13 Appendix 6-Chemical Safety Version#: 2.0
Page 10 of 12
SMILE
Johns Hopkins University
Baltimore, MD USA
2. them of the spill at [ number ] and evacuate the area.
4.
Seek medical attention.
XIV. DISPOSAL OF HAZARDOUS MATERIALS
1.
Chronic exposure and absorption of mercury may lead to a metallic taste in the mouth, a "lead line" (grey line) around gums, and neurological problems (irritable, hyper-reflexic, comatose).
Excess hazardous material must be disposed of in accordance with local regulations. Unwanted chemicals must be disposed through [ indicate chemical disposal process ]
Materials in any of the following categories must be disposed of as hazardous materials:
Ignitable - any substance with a flash point below 60 ° C (140 ° F)
Corrosive - any substance with pH of less than or equal to 2.0 or greater than or equal to
12.5.
Reactive - any substance which is unstable, reacts violently with water, forms potentially explosive mixtures with water, generates toxic gases, vapors or fumes when mixed with water or exposed to a pH between 2.0 and 12.5, or capable of detonation or explosive decomposition or reaction.
Toxic - any substance which contains any of the compounds listed by the EPA under the
Resource Conservation and Recovery Act at or greater than the listed concentration.
Specific chemicals - any substance containing an EPA listed compound.
3. C hemicals must be properly identified before proper disposal. “Unknown” materials cannot be disposed until they have been properly characterized with appropriate documentation.
4. [ Indicate any special disposal procedures, contacts or phone numbers ].
XV. CARCINOGENS - Specific regulations have been established by OSHA regarding the handling of certain compounds designated as carcinogenic. An inventory of all such materials must be maintained and specific protective measures observed.
1. Substances confirmed by OSHA to be carcinogenic to humans and require special precautions:
4-Aminodiphenyl, Skin bis(Chloromethyl) ether
4-Nitrodiphenyl, skin
β-Naphthylamine
Arsenic, elemental and inorganic compounds (except Arsine)
Asbestos (all forms)
Benzidine, Skin
Benzene - skin
Chromite ore processing (chromate)
Coal tar pitch volatiles as benzene solubles insoluble and water-soluble CrVi compounds,
NDC
Nickel, insoluble and soluble compounds
Vinyl chloride zinc chromates
2. Suspected human carcinogens:
1,1 - Dimethylhydrazine, Skin Chlormethyl methyl ether
1,3 - Butadiene Chloroform
1,4 - Dichloro - 2 - butene, skin Chrysene
2-Nitropropane Dichloromethane
Methulene chloride
Methyl hydrazine, Skin
Methyl iodide, Skin
N-Nitrosodimethylamine,
Skin
Fac1.0-13 Appendix 6-Chemical Safety Version#: 2.0
Page 11 of 12
3.
SMILE
Johns Hopkins University
Baltimore, MD USA
3,3' - Dichlorobenzidine, Skin Dimethyl sulfate, Skin
4 - vinul cyclohexene
4,4' - Methylene bis (2-
Dimethylcarbamoyl chloride
Dinitrotoluene chloroaniline)
4,4' - Methylene dianiline, Skin Epichlorohydrin
β-Propiolactone Ethul achylate
Acrylamide - Skin Ethul bromide, skin
Acrylonitrile, Skin Ethulene dibromide, skin
Antimony Trioxide production Ethylene oxide
Benz [a] anthracene
Benz [b] fluoranthene
Formaldehyde
Hexachlorobutadiene, Skin
Benzo(a)pyrene Hexachloroethane, skin
Beryllium and compounds
Cadmium elemental and compounds, as cd.
Calcium chromate
Hexamethyl phosphoramide,
Skin
Hydrazine, Skin
Lead chromate as Cr and Pb
N-Phenyl-beta-naphthylamine
O-Toluidine, Skin p-Toluidine, Skin
Phenylenediamine
Phenylhydrazine, Skin
Propane sultone
Propyleneimine, Skin
Strontium chromate
Tetranitromethane
Vinyl bromide
Vinyl chclohexene dioxide,
Skin xylicline (mixed isomers), skin
Zinc chromate
The above list of carcinogens is extracted from the lists provided by Chemical Threshold Limit
Values Committee of the American Conference of Governmental Industrial Hygienist's. If your laboratory has any of the above substances, please check with "Right To Know" list for specific recommendations on how to deal with any emergencies.
Fac1.0-13 Appendix 6-Chemical Safety Version#: 2.0
Page 12 of 12