Hazards – Appendix O
advertisement

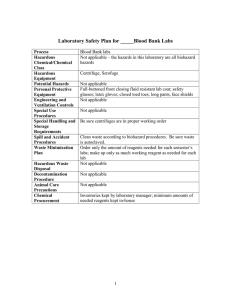
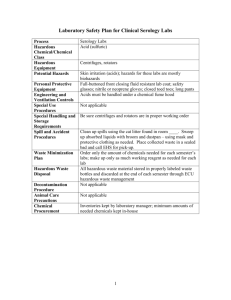
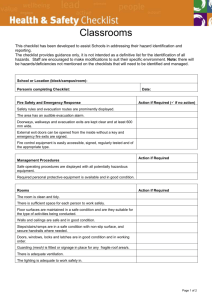
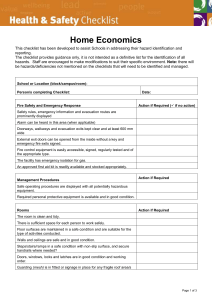
O SECTION ANIMAL EXPERIMENTS INVOLVING HAZARDOUS AGENTS Complete only if applicable to your project. This portion of the protocol is for applications using hazardous agents IN VIVO such as radioisotopes, infectious agents, carcinogens or toxic chemicals. Because many of the questions are “agent-specific”, unless toxicity/treatment/etc. are the same, please duplicate this form for each agent. O.1. Study Involves: Radioactive Materials or Radiation Infectious Agents (pathogenic to human or animal) Acute Toxins Known or suspect chemical carcinogens or mutagens Recombinant or non-Recombinant DNA/RNA Other (Describe) O.2. STATUS OF REVIEW by the appropriate Hazards Committee (Attach approval(s) (If pending approval, a copy must be submitted as soon as approval is obtained) Committee Name(s) Approval date(s) O.3. Identify agent O.3.a. Agent/Chemical/Isotope O.3.b. Species O.3.c. Dose (Max Vol) O.3.d. Route O.3.e. Needle Size O.3.f. Frequency YES NO O.4. Are there risks to humans? ((If YES, complete the following) O.4.a. Method of exposure How could humans be exposed (e.g., excreted in animal’s feces or urine. Airborne. Only on direct contact with contaminated animal, etc.)? O.4.b. O.4.c. O.4.d. Signs/Symptoms Treatment Protection (Personal Protective Devices) YES NO O.5. Are there risks to other animals in the room or animal facility? (If YES, complete the following) Can other animals in the same room or in the facility become exposed (infected, contaminated, affected, etc.)? O.5.a. Method of exposure How could the other animals in the facility be exposed (e.g. excreted in feces or urine. Airborne. Only on direct contact with contaminated animal, etc.)? O.5.b. O.5.c. O.5.d. Signs Treatment Protection O.6. Describe experimental procedures involving hazardous agents YES O Section Hazards Page 1 of 2 NO O.7. Is the duration of hazardous agents use the same as the total project duration? If different, state approximate time period. Start Date Stop Date YES NO YES NO YES NO O.8. Is there special animal care required relating to the use of hazardous materials? (If YES, please describe) O.9. Are there special containment facility requirements? (If YES, complete below) O.9.a. Biohazard Room Outside the Animal Facility O.9.b. Biohazard Room Within the Animal Facility O.9.c. Other Containment Requirements (Please Describe) O.10. Are there waste and animal disposal requirements? (If YES, describe) O Section Hazards Page 2 of 2