Charles Law - schoolphysics
advertisement

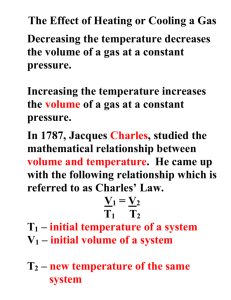
The expansion of a gas – absolute zero and Charles’ Law The air in a hot air balloon expands when it is heated so lowering its density and helping the balloon to rise A pressurised gas canister will explode if heated over a fire and so can be very dangerous When a tin full of air is heated strongly the lid will be blown off When the petrol-air mixture in the cylinder of an engine is ignited it expands If you heat a blown up balloon above a Bunsen flame it will burst These effects are all happen because a gas tries to expand when it is heated. When the gas is heated the molecules in the gas move round faster. They are being given energy in the form of heat and this appears as the extra kinetic energy of the gas molecules. The molecules bang into each other and into the walls of the container more violently and this increases the pressure and so the gas tries to expand. If we allow the gas to expand the pressure can be kept constant and we can investigate the connection between the temperature of the gas and its volume. This can be done using the simple apparatus shown in the diagram. The tube and the thermometer are immersed in water which is then heated. The trapped air in the tube expands and the volume at different temperatures can be recorded. If a graph of volume against temperature is plotted you should get a result similar to graph 1. Volume Temperature (oC) (Notice that the pressure of the trapped air stays the same – atmospheric pressure) (For full details of this experiment see 14-16/Gases/Experiments/Charles Law) However if we draw the line back it to the point where the theoretical volume of the gas is zero it will cut the temperature axis at a point well below 0oC. We call this point ABSOLUTE ZERO. We could then measure all temperatures by starting at this point and if we do then we will get a graph like graph 2. If the experiment has been done really carefully absolute zero turns out to be about –273oC. This is the coldest that it is possible to get. Volume Temperature (K) Absolute zero 1 The Kelvin (or absolute) temperatue scale It is useful to define a new scale of temperature starting at absolute zero. The size of the degrees is the same as those for the Celsius scale but the numbers are different. We call this scale the ABSOLUTE or KELVIN temperature scale. Temperatures on this scale are given the symbol K. So for example absolute zero (-273oC) become 0 K on the Kelvin scale. The freezing point of water (0oC) becomes 273 K on the Kelvin scale. The boiling point of water becomes 373 K on the Kelvin scale and so on. To change from Celsius to Kelvin you simply ADD 273 and to change from Kelvin to Celsius you simply take 273 away from the temperature. When working with gas equations like the one below you must ALWAYS use the Kelvin scale. In deep space far from any stars the temperature is still 3K or –270oC!). Charles’ Law The equation for the line was first suggested by the French Physicist Jacques Charles in 1787. It states that for an ideal gas: Volume of the gas/Absolute temperature of the gas = constant As long as the pressure of the gas is kept constant. If the gas is heated so that its volume changes from volume 1 to volume 2 while its temperature changes from temperature 1 to temperature 2 then: Volume 1/Temperature 1 = Volume 2/Temperature 2 Example problem A sample of air with a volume of 3 m3 at 27oC (300K) is heated to a temperature of 100oC (373K). If the pressure of the air is kept the same what is the new volume of the sample of air. 3/300 = V2/373 Therefore: V2 = 373/100 = 3.73 m3 Note: An ideal gas is one which cannot be liquefied by simply increasing the pressure. The molecules of the gas are supposed to exert no force on each other. If you want to learn more about ideal gases then have a look at the 16-19 text section. 2

![Temperature Notes [9/22/2015]](http://s3.studylib.net/store/data/006907012_1-3fc2d93efdacd086a05519765259a482-300x300.png)