Periodic Table Worksheet
advertisement
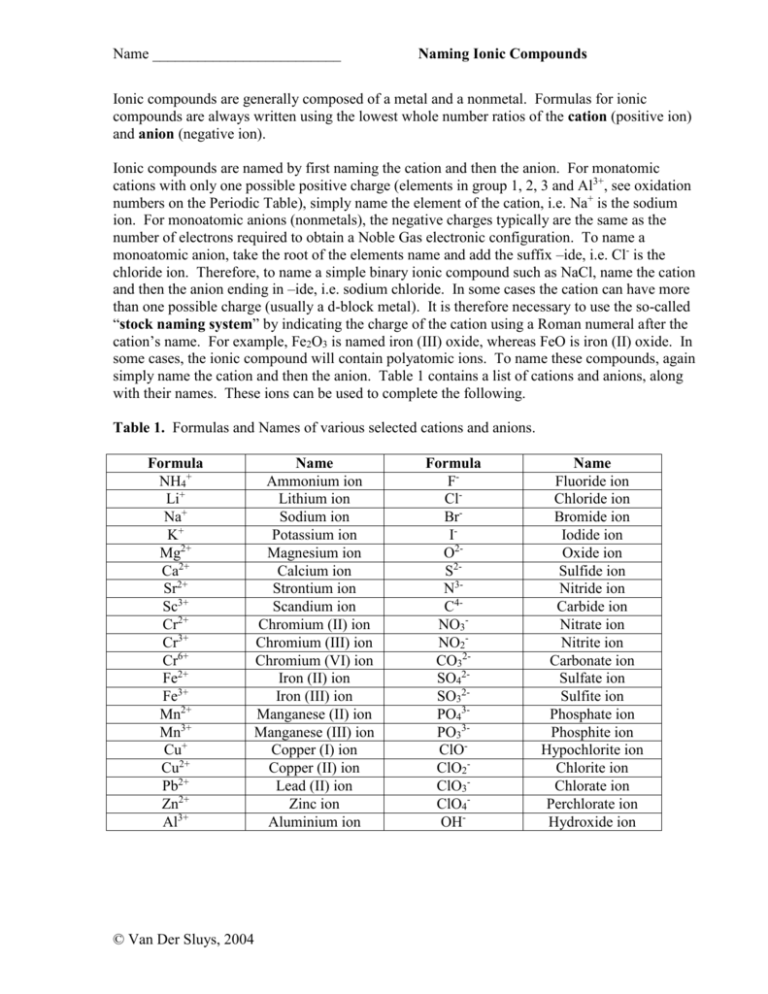
Name _________________________ Naming Ionic Compounds Ionic compounds are generally composed of a metal and a nonmetal. Formulas for ionic compounds are always written using the lowest whole number ratios of the cation (positive ion) and anion (negative ion). Ionic compounds are named by first naming the cation and then the anion. For monatomic cations with only one possible positive charge (elements in group 1, 2, 3 and Al3+, see oxidation numbers on the Periodic Table), simply name the element of the cation, i.e. Na+ is the sodium ion. For monoatomic anions (nonmetals), the negative charges typically are the same as the number of electrons required to obtain a Noble Gas electronic configuration. To name a monoatomic anion, take the root of the elements name and add the suffix –ide, i.e. Cl- is the chloride ion. Therefore, to name a simple binary ionic compound such as NaCl, name the cation and then the anion ending in –ide, i.e. sodium chloride. In some cases the cation can have more than one possible charge (usually a d-block metal). It is therefore necessary to use the so-called “stock naming system” by indicating the charge of the cation using a Roman numeral after the cation’s name. For example, Fe2O3 is named iron (III) oxide, whereas FeO is iron (II) oxide. In some cases, the ionic compound will contain polyatomic ions. To name these compounds, again simply name the cation and then the anion. Table 1 contains a list of cations and anions, along with their names. These ions can be used to complete the following. Table 1. Formulas and Names of various selected cations and anions. Formula NH4+ Li+ Na+ K+ Mg2+ Ca2+ Sr2+ Sc3+ Cr2+ Cr3+ Cr6+ Fe2+ Fe3+ Mn2+ Mn3+ Cu+ Cu2+ Pb2+ Zn2+ Al3+ Name Ammonium ion Lithium ion Sodium ion Potassium ion Magnesium ion Calcium ion Strontium ion Scandium ion Chromium (II) ion Chromium (III) ion Chromium (VI) ion Iron (II) ion Iron (III) ion Manganese (II) ion Manganese (III) ion Copper (I) ion Copper (II) ion Lead (II) ion Zinc ion Aluminium ion © Van Der Sluys, 2004 Formula FClBrIO2S2N3C4NO3NO2CO32SO42SO32PO43PO33ClOClO2ClO3ClO4OH- Name Fluoride ion Chloride ion Bromide ion Iodide ion Oxide ion Sulfide ion Nitride ion Carbide ion Nitrate ion Nitrite ion Carbonate ion Sulfate ion Sulfite ion Phosphate ion Phosphite ion Hypochlorite ion Chlorite ion Chlorate ion Perchlorate ion Hydroxide ion Name _________________________ Naming Ionic Compounds Fill in the blank spaces of the following table. Formula Cation Anion Ca2+ CO32- Name of ionic compound KCl Iron (II) sulfate Fe2(SO4)3 Mg2+ ClChromium (III) Chloride Zn3(PO4)2 Al3+ OHammonium nitrate (NH4)2SO4 Na+ FCopper (II) sulfate Ca(NO3)2 Zn2+ © Van Der Sluys, 2004 N3- Name _________________________ Naming Ionic Compounds Answers Formula Cation Anion Name of ionic compound KCl K+ Cl- Potassium chloride CaCO3 Ca2+ CO32- Calcium carbonate FeSO4 Fe2+ SO42- Iron (II) sulfate Fe2(SO4)3 Fe3+ SO42- Iron (III) sulfate MgCl2 Mg2+ Cl- Magnesium chloride CrCl3 Cr3+ Cl- Chromium (III) Chloride Zn3(PO4)2 Zn2+ PO43- Zinc phosphate Al(OH)3 Al3+ OH- Aluminum hydroxide NH4NO3 NH4+ NO3- ammonium nitrate (NH4)2SO4 NH4+ SO42- Ammonium sulfate NaF Na+ F- Sodium fluoride CuSO4 Cu2+ SO42- Copper (II) sulfate Ca(NO3)2 Ca2+ NO3- Calcium nitrate Zn3N2 Zn2+ N3- Zinc nitride © Van Der Sluys, 2004 Name _________________________ © Van Der Sluys, 2004 Naming Ionic Compounds







