Practice Test 2 Ch. 11 (Thermochemistry)
advertisement
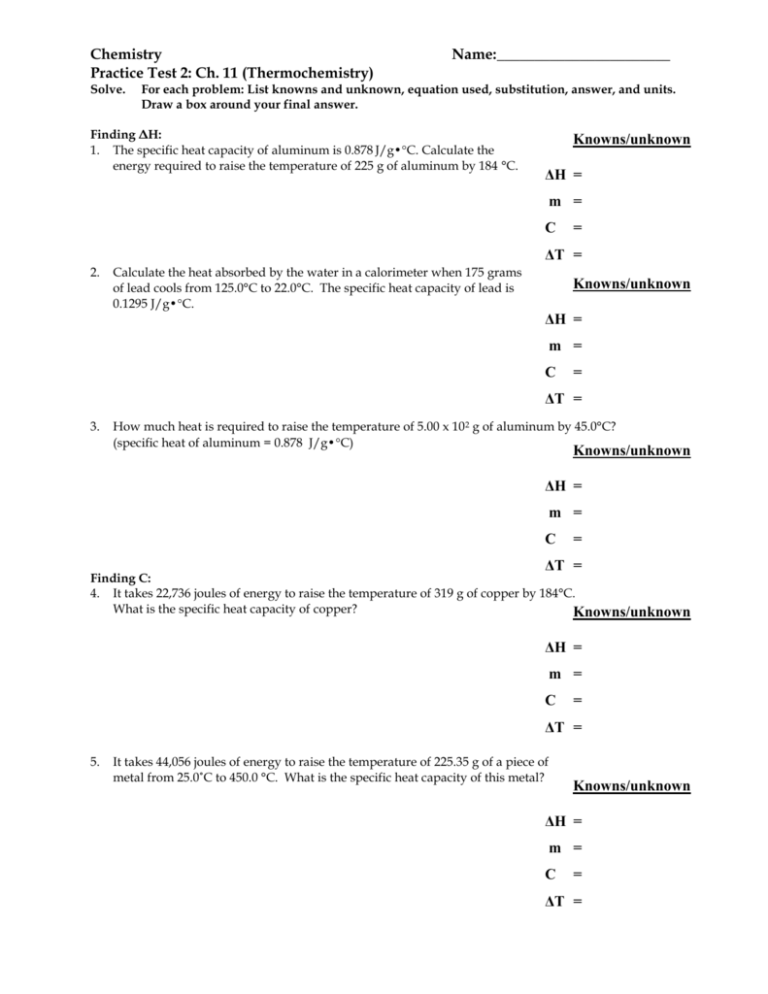
Chemistry Practice Test 2: Ch. 11 (Thermochemistry) Solve. Name: _______________________ For each problem: List knowns and unknown, equation used, substitution, answer, and units. Draw a box around your final answer. Finding ΔH: 1. The specific heat capacity of aluminum is 0.878 J/g•C. Calculate the energy required to raise the temperature of 225 g of aluminum by 184 °C. Knowns/unknown ΔH = m = C = ΔT = 2. Calculate the heat absorbed by the water in a calorimeter when 175 grams of lead cools from 125.0°C to 22.0°C. The specific heat capacity of lead is 0.1295 J/g•C. Knowns/unknown ΔH = m = C = ΔT = 3. How much heat is required to raise the temperature of 5.00 x 10 2 g of aluminum by 45.0°C? (specific heat of aluminum = 0.878 J/g•C) Knowns/unknown ΔH = m = C = ΔT = Finding C: 4. It takes 22,736 joules of energy to raise the temperature of 319 g of copper by 184°C. What is the specific heat capacity of copper? Knowns/unknown ΔH = m = C = ΔT = 5. It takes 44,056 joules of energy to raise the temperature of 225.35 g of a piece of metal from 25.0˚C to 450.0 °C. What is the specific heat capacity of this metal? Knowns/unknown ΔH = m = C = ΔT = Finding ΔT and Tf 6. Assume 444.55 joules of heat are added to 8.50 g of water originally at 25.0°C. What would be the final temperature of the water? (Note: Find T first then find the final temperature) Note: The specific heat capacity of water = 4.184 J/g•C. Knowns/unknown ΔH = m = C = ΔT = Ti = Tf = Lab 16 Problem: 7. Find the specific heat capacity of iron if a 125.0 g sample of iron with an initial temperature of 98.0°C is placed into 132.85 g of water with an initial temperature of 34.0 °C. The final temperature of the water and the iron is 40.0 °C. Remember the specific heat capacity of water is 4.184 J/g˚C. Water ΔH = m = C = ΔT = Metal ΔH = m = C = ΔT = 8. Find the standard heat of formation for the following chemical reaction. Use the table provided. 2 C2H6 (g) + 7 O2 (g) → 4 CO2(g) + 6 H2O (g) H° = Hf° (products) - Hf° (reactants) Substance Hf° (kJ/mol) C2H6 (g) O2 (g) CO2(g) H2O(g) - 84.7 0.0 - 394 - 242 9. Heat of Reaction: How much heat does it take to decompose when 250.50 g of Al2O3 according to the equation below? 2 Al2O3 (s) → 4 Al(s) + 3 O2 (g) H = 3352 kJ 10. Heat of Solution: Determine the heat of solution when 152.25g of CaCl2 is dissolved in water? Hsoln = - 82.8 kJ/mol 11. Heat of Combustion: Analyze the reaction: 2 C4H10 (g) + 13 O2 (g) → 8 CO2(g) + 10 H2O (g) H = - 5718 kJ. How much heat is produced when 4.50g of butane, C4H10 (at STP) is reacted with excess O2? 12. (Hess’s Law) Rewrite the equations to calculate ΔH for the reaction below: Calculate the enthalpy change (H) in kJ for the following reaction. N2(g) + 3 H2O(g) 1.5 O2(g) + 2 NH3(g) Use the enthalpy changes from the following equations. Reaction 1: Reaction 2: N2 (g) 3 H2(g) + + 3 H2(g) 1.5 O2(g) 2 NH3(g) 3 H2O(g) H = - 92 kJ H = - 726 kJ H = _________ 13. (Show All Work) How much heat is absorbed by 225.0 g of ice at - 25.0 °C to steam at 130.0 °C? (Hfus= 6.01 kJ/mol , Hvap = 40.7 kJ/mol , Cice= 2.1 J/(g °C), Cliquid= 4.184 J/(g °C), and Csteam= 1.7 J/(g°C)) Hint: Calculate the energy for each phase then find the total energy. Finding ΔH: (similar to Questions 1-3 on the 1st page) Solid phase: (Temperature increases from -25.0 C to 0C) Heat of Fusion Problem: (Use unit conversions with ΔHfus) Melting: (Temperature stays at 0 C) Finding ΔH: (similar to Questions 1-3 on the 1st page) Liquid phase: (Temperature increases from 0 C to 100 C) Heat of Vaporization Problem: (Use unit conversions with ΔHvap) Boiling: (Temperature stays at 100 C) Finding ΔH: (similar to Questions 1-3 on the 1st page) Gas phase: (Temperature increases from 100 C to 130.0 C) Total energy: