updated research
advertisement

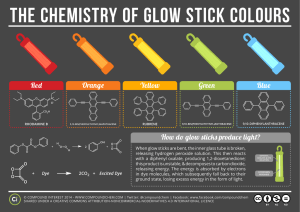
GLOW STICKS ~ research What is a glow stick? o A glow stick is a single – use translucent plastic tube containing isolated substances which when combined make light through a chemical reaction. What chemicals are used in glow sticks? o diphenyl oxalate a solid ester whose oxidation products are responsible for the chemiluminescence in a glowstick. o flourescent dye solution o hydrogen peroxide an oxidizer commonly used as a bleach ; clear liquid; appears colorless in dilute solution How does it work? o Glass capsule divides solutions o After glass capsule is broken and the solutions mix, it produces a chemical reaction and the glow stick glows. Why does it glow? o by mixing the peroxide with the phenyl oxalate ester, a chemical reaction occurs; the ester is oxidized, yielding two molecules of phenol and one molecule of peroxyacid ester; the peroxyacid decomposes spontaneously to carbon dioxide, releasing energy that excites the dye, which then relaxes by releasing a photon photon – a packet of light or electromagnetic radiation; also called quantum of light wavelength of photon depends on structure of the dye What different fluorophores are used to emit different colors? o 9, 10-diphenylanthracene (DPA) = blue light o 1-chloro(DPA) & 2-chloro(DPA)= blue-green light; dihydro(DPA) = purple o 1-chloro-9(BPEA) = yellow-green light o 2-chloro-9(BPEA) = green light o 1,8-dichloro-9(BPEA) = yellow light o 2,4-di-tert-butylphenyl 1,4,5,8-tetracarboxynaphthalene diamide = deep red light + DPA = white or hot-pink light o 5,12-bis(phenylethynyl)naphthacene = orange light o Rubrene = orange-yellow light