Glow Sticks - WordPress.com
advertisement

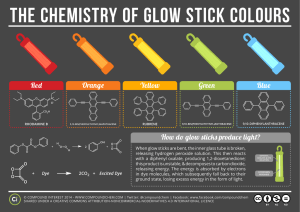
McCall Iorg Chemistry 1010 May 1, 2013 Reacting with Chemistry Driving to a diner with my best friend, I had forgotten all about chemistry from earlier that day. Along with the idea of coming up with an interesting topic to present at the end of the semester and it relating to chemistry. Finding a topic, let alone an interesting one in chemistry, was hard enough. But that was at the end of semester and right here and now I was prepared to have a good time. After we left the diner with our little trinkets of glow sticks, bubble gum, and stickers, we were on our way home. “Pop open the glow sticks”, I said as I shifted into reverse. She hands me one, I set it in my lap, and she took the other. Crack. She starts from left to right bending the glow stick and watching it come to life with the color or neon, yellow. I look over and realize….that’s my idea for the presentation in chemistry. I didn’t know how glow sticks worked, but I know that I wanted to find out. Now it’s true that not many people often wonder what chemicals it takes to make the glow sticks to “glow” because it’s not a huge, flashy reaction, but now you’re wondering what makes it happen because you don’t know either. Chemical reactions, though small, still go through a lot. A glow stick is a self-contained, short-term light-source. It consists of a translucent plastic tube containing isolated substances that, when combined, make light through chemiluminescence, so it does not require an external energy source. They are waterproof, do not use batteries, generate negligible heat, are inexpensive, and are reasonably disposable. The light cannot be turned off, and can be used only once. Glow sticks are often used for recreation, but may also be relied upon for light during military, police, fire, or EMS operations. Glow sticks are actually a big part in the application of a natural phenomenon in chemistry called luminescence. Luminescence is any emission of light that is not caused by heating, first introduced by Eilhard Wiedemann. Wiedemann thought luminescence a form of "radio-luminescence", although it is today considered to be separate since it involves more than electromagnetic radiation. Other examples include: televisions, neon lights and glow-in-the-dark stickers. There is no one, single strict way to create a light/glow stick. What you need is two solutions separate from one another. One being hydrogen peroxide by itself and the other being phenyloxalate ester attached together with a fluorescent dye. On the outside is a plastic shell of a glow stick, and within lies a glass tube. The phenyl oxalate ester and dye solution fills most of the plastic outer shell itself. The hydrogen peroxide solution, called the activator, is contained in a small, fragile glass vial within the outer shell of the glow stick. When you bend the bend the plastic you are also breaking the glass. Thus the cracking noise. In chemistry terms the reaction that gives off light is called chemiluminescence. Breaking the barrier and causing the hydrogen peroxide and phenyloxalate ester to react. The reaction causes excitement among the electrons and enough energy in the fluorescent dye. Making those same electrons skip to a higher energy level, back down, and create that light behind the “glow” stick. To make your own is harder and a more expensive, but can be done. First, you’d have to start with diethyl phthalate. Keep in mind that you can’t use water because some of the chemicals don’t’ work in water. From there you selected the dye you want. In the video I saw he chose to use 9, 10-bis (phenylethynyl) anthracene. It look’s orange in the solid state, but dissolves to a green color after mixing these two solutions together. That’s what is going to make it give the color of green when it “glows”. The next ingredient is the most important: bis (2, 4, 6-trichlorophenyl) oxalate, also known as TCPO. This gives the right type of energy for this chemical reaction to generate light with a fluorescent dye. In the video, he then adds sodium acetate as a base because this reaction occurs better in acidic conditions. Sodium bicarbonate also works just as well if you don’t have sodium acetate. The final element he puts in is hydrogen peroxide. It reacts with the TCPO to decompose it and bring the chemical energy to transfer to the dye to make the stick “glow”. You might wonder where the bonding within the reaction comes from…When diphenyl oxalate reacts with hydrogen peroxide (H2O2); it is oxidized to give phenol and cyclic peroxide. The peroxide reacts with a molecule of dye to give two molecules of carbon dioxide (CO2) and in the process; an electron in the dye molecule is promoted to an excited state. When the excited (high-energy) dye molecule returns to its ground state, a photon of light is released. The reaction is pH-dependent. When the solution is slightly alkaline, the reaction produces a brighter light. Now some wonder what might happen if you remove the dye and just mix the chemicals. I can tell you now that it won’t work. Because while the chemicals create energy they need to react the dye to create the light. Fluorescent dyes work differently than regular absorption dyes. The regular absorption based dyes absorb only particular colors and then reflecting or passing through other colors. The florescent dyes also do this, but can also generate light by converting light with high energy into lower energy. Not only do the electrons skip to different energy levels, but you can control how fast or slow you make this reaction happen. If you heat the solution the extra energy will accelerate the reaction, and the stick will glow brighter, but for a shorter amount of time. But if you cool the light stick, the reaction will slow down, and the light will dim. The best way to preserve your glow stick for any reason put it in the freezer -- it won't stop the process, but it will drag out the reaction considerably. To simply summarize the reaction: The hydrogen peroxide oxidizes the phenyl oxalate ester, to form phenol and an unstable peroxyacid ester. The unstable peroxyacid ester decomposes, resulting in phenol and a cyclic peroxy compound. The cyclic peroxy compound decomposes to carbon dioxide. This decomposition reaction releases the energy that excites the dye. Yes. You’ve just learned what it takes to make a glow stick. But what good does that do for you right? It’s not the question of what this new knowledge does for you, but more of the question when do you see it being used? Little kids use them when they go trick-or-treating. Divers use them as guidance through murky waters. Campers that have the stealth of invisibility but can see everything. And of course for all of you who have that crazy side…parties where everything is in the dark. Chemistry reactions don’t mean that it has to be big and spontaneous. Mine was actually quite small, but big in reaction. A lot happened to make that reaction happen, but didn’t explore or cause a flame. You don’t have to look very hard for chemistry in your life. It is right in front of you, so let it find you.