Heat of Solution of NH4NO3 Lab: Endothermic Reactions
advertisement

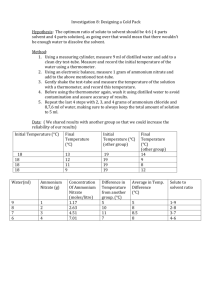
Heat of Solution of NH4NO3 Chemical and physical changes are usually accompanied by the liberation or absorption of energy. If energy is evolved, the reaction is said to be EXOTHERMIC. If the energy is absorbed, the reaction is ENDOTHERMIC. Heat is a form of energy, and is measured in Joules (J). Energy may be transformed from one kind to another within an isolated (or closed) system but the total energy does not change. If the change in energy of a system can be measured and if this change is due solely to a chemical reaction, then the energy change must be equal to that of the chemical reaction itself. A Styrofoam cup shall be used as a calorimeter and the change in energy of the system will be measured by recording the temperature of a given weight of water before and after the reaction occurs. The specific heat capacity of water (i.e., the energy required to raise the temperature 1o C of 1 gram of the material) is very nearly 4.2 J/g/oC for temperatures between 0o C and 100o C. If a calorimeter contained 100 grams of water at 23.0o C initially and after the reaction took place there were still 100. grams of water and the temperature was now 30.0o C, the energy liberated in the reaction would be Q (J) = mass (g) x t (oC) x cp (J/goC) 2940 J = 100. g x 7.00o C x 4.2J/g/oC This calculation assumes that no energy was required to raise the temperature of the calorimeter itself and that no energy was lost, or gained, by the calorimeter during the experiment. Procedure: 1. Use a graduated cylinder 10 measure 100 ml of water and transfer the water to the Styrofoam cup. Record the temperature of the water. 2. Weigh accurately, between two and three grams of NH4NO3. 3. Add the NH4NO3 to the water, continually swishing the cup gently, and with constant observation of the temperature until it remains constant for about 15-20 seconds. 4. Record this temperature. 5. After completing all data collection for this experiment, move on to the second experiment Data Table: mass of 75.0 mL of water ______ mass of NH4NO3 ______ initial temperature of water ______ final temperature of solution ______ temperature change ______ Questions: 1. Is the dissolution of the NH4NO3 in water an exothermic or endothermic process? ? 2. Assuming 1 mL of water has a mass of 1 gram and the specific heat of the dilute solution is the same as water, calculate the number of joules involved in the dissolution of the NH4NO3. (use actual mass of H2O) # of joules = 75 g x temperature change x 4.185 J/gºC NH4NO3 ______________ J 3. How many moles of each substance were dissolved? # of moles = grams dissolved Molar mass NH4NO3 _____________ mole Calculate the number of joules that would be involved if one mole of the substance were dissolved in water. 4. # of J = # of J (see question 2 above) mole # of moles (see question 3 above) NH4NO3 ____________ J/mol