File
advertisement
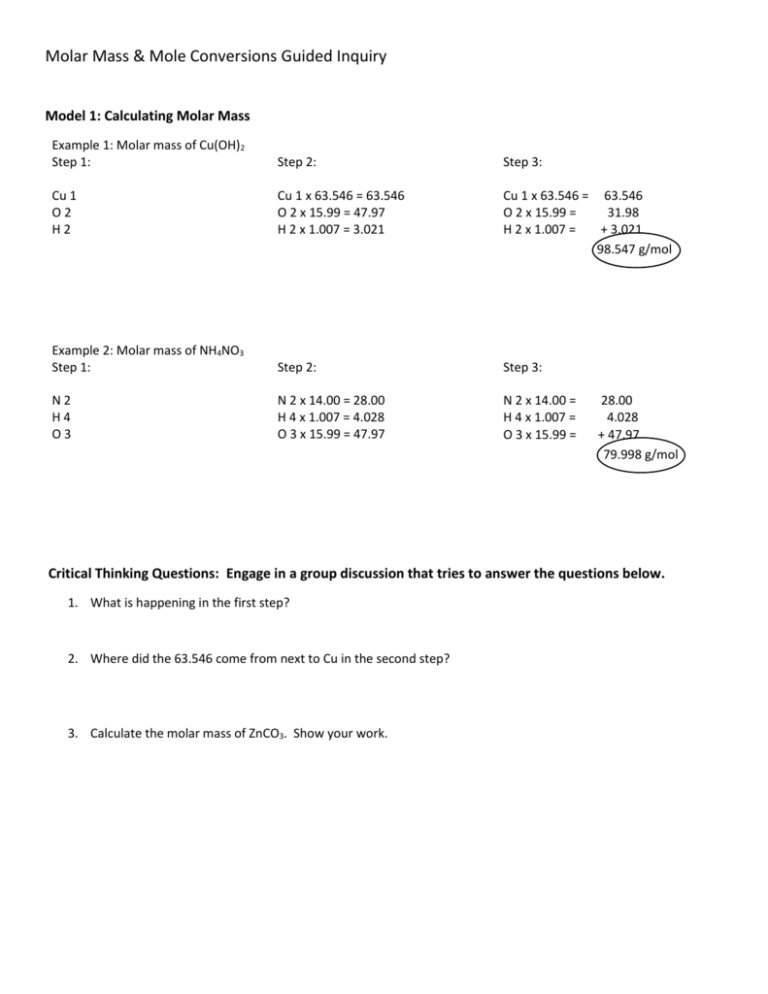
Molar Mass & Mole Conversions Guided Inquiry Model 1: Calculating Molar Mass Example 1: Molar mass of Cu(OH)2 Step 1: Step 2: Step 3: Cu 1 O2 H2 Cu 1 x 63.546 = 63.546 O 2 x 15.99 = 47.97 H 2 x 1.007 = 3.021 Cu 1 x 63.546 = 63.546 O 2 x 15.99 = 31.98 H 2 x 1.007 = + 3.021 98.547 g/mol Example 2: Molar mass of NH4NO3 Step 1: Step 2: Step 3: N2 H4 O3 N 2 x 14.00 = 28.00 H 4 x 1.007 = 4.028 O 3 x 15.99 = 47.97 N 2 x 14.00 = H 4 x 1.007 = O 3 x 15.99 = 28.00 4.028 + 47.97 79.998 g/mol Critical Thinking Questions: Engage in a group discussion that tries to answer the questions below. 1. What is happening in the first step? 2. Where did the 63.546 come from next to Cu in the second step? 3. Calculate the molar mass of ZnCO3. Show your work. Model 2: Converting Between Grams and Moles Example 1: Determine the number of moles in 24 grams of copper (II) hydroxide, Cu(OH)2. 24 g Cu(OH)2 1 mol Cu(OH)2 98.547 g Cu(OH)2 = 0.243 mol Cu(OH)2 Example 2: Determine the number of grams in 0.52 moles of ammonium nitrate, NH4NO3. 0.52 mol NH4NO3 79.998 g NH4NO3 1 mol NH4NO3 = 0.243 g NH4NO3 Critical Thinking Questions: Engage in a group discussion that tries to answer the questions below. 1. What is the number (98.574 g) on the bottom of the first chart called? 2. Units, like grams and moles, cancel out when they are found on the top and bottom of the chart. Cross out any units that cancel in both examples. 3. What do you notice about the units of the answer and the units within the chart? Where are the units for the answer found in the chart? 4. Write two or three guidelines for how to set up the charts. 5. Calculate the number of grams in a 5.3 moles sample of CO2. Set up the chart to show your work. Model 3: Converting Between Particles and Moles Example 1: Determine the number of moles in a sample containing 2.5x1024 copper atoms. 2.5x1024 Cu atom 1 mol Cu 6.02x1023 atom Cu = 4.15 mol Cu Example 2: Determine the number of molecules of ammonium nitrate in a 2.5 mole sample. 2.5 mol NH4NO3 6.02x1023 molecules NH4NO3 1 mol NH4NO3 = 1.51 molecules NH4NO3 Critical Thinking Questions: Engage in a group discussion that tries to answer the questions below. 1. In Model 2, the molar mass was used to convert between grams and moles. What number is used to convert between particles (atoms or molecules) and moles? 2. Calculate the number of molecules in a 5.2 mole sample of water. Set up the chart to show your work. Making Sense: Engage in a group discussion that tries to answer the questions below. 1. Condense all of the rules you’ve written and discovered above into four or five short bullet points explaining how to convert between grams, moles and particles. Conversion factors are used to convert between different units. When converting between grams and moles the conversion factor used is the molar mass. When converting between moles and particles, the conversion factor is Avogadro’s number, 6.02x1023. The conversion factor used to convert between moles and liters is 22.4 liters. Use this information and the rules you wrote above to solve the following problems. 2. How many moles are in a 7.3 liter sample of hydrogen gas? Show your work. 3. How many grams are in a 5.4 mole sample of CBr4? Show your work. 4. A student uses 5.21x1027 AgNO3 molecules during a lab. How many moles is this? Show your work.







