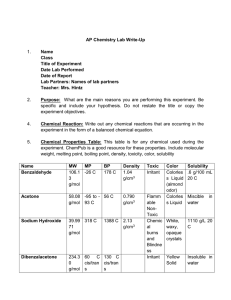
Synthesis of Dibenzalacetone Microscale Procedure
advertisement

Synthesis of Dibenzalacetone: Microscale Procedure IN THIS EXPERIMENT an ethanolic solution of acetone and benzaldehyde is to aqueous sodium hydroxide. The product, dibenzalacetone, crystallizes after minutes. The product is filtered from the mixture, washed, pressed dry, and realized from an ethanol-water mixture. This very important reaction is easily carried out. Into a 10 X 100-mm reaction tube place 2 mL of 3 M sodium hydroxide solution. To this solution add 1.6 mLof95% ethanol and 0.212 g of benzaldehyde. Then add 0.058 g of acetone to the reaction mixture. Alternatively, your instructor may provide a solution that contains 58 mg of acetone in 1.6 mL of ethanol the tube immediately and shake the mixture vigorously. The benzaldehyde, initially insoluble, goes into solution, resulting in a water-clear, pale-yellow solution. After a minute or so it suddenly becomes cloudy, and a yellow precipitate product forms. Continue to shake the tube from time to time for the next 30 min. If the product fails to crystallize, open the tube and scratch the inside of the tube with a glass rod. Remove the liquid from the tube using a Pasteur pipette by squeezing the bulb of the pipette, pressing the tip against the bottom of the tube, and bringing the liquid into the pipette, leaving the crystals in the tube (Pig. 37.1). Add 3 mL of water, cap, and shake the tube vigorously. Remove the wash liquid as before and wash the crystals twice more with 3-mL portions of water. After the final washing, add 3 mL of water to the tube and collect the crystals on a Hirsch funnel using vacuum nitration. Use the filtrate to complete the transfer of the crystals. Squeeze the product between sheets of filter paper to dry it and then recrystallize the crude dibenzalacetone from a 70:30 ethanol-water mixture. During the recrystallization process (see Chapter 4), insert a wooden boiling stick to promote even boiling when heating the solute in the solvent. Remove the tube from the hot sand bath and place it in an insulated container to cool slowly to room temperature. Should the product separate as an oil, obtain a seed crystal, heat the solution to dissolve the oil, and add the seed crystal as the solution cools. If the product continues to oil out, add a bit more ethanol. After cooling the tube for several minutes in ice, collect the product by removing the solvent with a pipette (Fig. 37.1) and washing once with about 0.5 mL of ice-cold 70% ethanol while the tube is in ice. Alternatively, collect the crystals on a Hirsch funnel (Fig. 37.2) or Wilfilter and wash once with ice-cold 70% ethanol. Dry the product under vacuum by attaching the tube to an aspirator (Fig. 37.3). Determine the melting point a pure sample will have a melting point between 110.5-112°C. The yield should be around 0.10 grams.