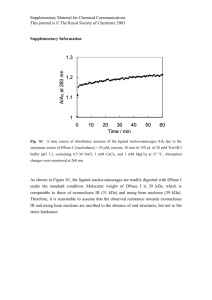
- Northumbria Research Link
advertisement

Discovery of a cell wall porin in the mycolic acid-containing actinomycete Dietzia maris Samaneh Mafakheri1, Iván Bárcena-Uribarri1, Nafiseh Soltan Mohammadi1, Narges Abdali1, Amanda L. Jones2, Iain C. Sutcliffe2, Roland Benz1* 1 School of Engineering and Science, Jacobs University Bremen, Campusring 1, D-28759 Bremen, Germany, and 2 Faculty of Health and Life Sciences, Northumbria University, Newcastle upon Tyne NE1 8ST, United Kingdom *Corresponding author: Roland Benz, School of Engineering and Science Jacobs-University Bremen, Campusring 1, D-28759 Bremen, Germany TEL: +49 421 200-3151 FAX: +49 421 200-3249 E-mail: r.benz@jacobs-university.de Running title: Cell wall porin of Dietzia maris Key words: Dietzia maris, porin, lipid bilayer, cell wall FEBS Journal format http://onlinelibrary.wiley.com/journal/10.1111/(ISSN)1742-4658 Abstract |D i e t z i a m a r i s m a n u s c r i p t 1 The cell wall of the Gram-positive mycolic acid-containing actinomycete Dietzia maris was found to contain a pore-forming protein, as observed from reconstitution experiments with artificial lipid bilayer experiments in the presence of cell wall extracts. The cell wall porin was purified to homogeneity using different biochemical methods and had an apparent molecular mass of about 120 kDa on tricine-containing sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE). The 120 kDa protein dissociated into subunits with a molecular mass of about 35 kDa when it was heated to 100 ºC. The 120 kDa protein formed ionpermeable channels in lipid bilayer membranes with a high single-channel conductance of about 5.8 nS in 1 M KCl. Channel-formation was very frequent and the lifetime of the pores was very long. Asymmetric addition of the cell wall porin to lipid bilayer membranes resulted in an asymmetric voltage-dependence. Zero-current membrane potential measurements with different salt solutions suggested that the porin of D. maris is cation selective because of negative charges localized at the channel mouth. Analysis of the single-channel conductance using non-electrolytes with known hydrodynamic radii indicated that the diameter of the cell wall channel was about 2.1 nm. The channel characteristics of the cell wall porin of D. maris were compared to those of other members of the mycolata. They share some common features because they are composed of small molecular mass subunits and form large and water-filled channels. Introduction |D i e t z i a m a r i s m a n u s c r i p t 2 Dietzia maris was originally known as Rhodococcus maris [1] but later was classified as a species of the new genus Dietzia. This genus has now been placed into the family Dietziaceae within the suborder Corynebacterineae of the Actinomycetales [2,3]. Dietzia maris is a Gram-positive, aerobic, non-spore-forming soil bacterium which forms cocci that can develop into short rods [4]. In addition to the possibility of playing a role in waste water treatment and its presence in activated sludge [5], it has been also reported that D. maris has a potential application in bioremediation of soil environments [6] and as a producer of the antioxidant canthaxanthin [7]. Dietzia sp. are only rarely reported as pathogens although they may often be misidentified, notably as rhodococci [2,8]. D. maris belongs to the mycolata, the widespread group of mycolic acid-containing actinomycetes [2,4,9]. These bacteria produce cell walls of a unique structure with low permeability that contains large amounts of lipids on top of the peptidoglycan layer in form of 2-branched, 3-hydroxylated fatty acids, the mycolic acids. The mycolic acids are covalently linked to the arabinogalactan attached to the murein of the cell wall [10,11]. There is some evidence suggesting that this structure has a low permeability because the mycolic acids represent a hydrophobic barrier [12-14]. This means that the mycolic acid layer has the same function as the outer membranes of Gram-negative bacteria, which contain porins for the passage of hydrophilic solutes. Accordingly, channel forming proteins with characteristics similar to porins of their Gram-negative counterparts have been identified to exist in the cell walls of some members of mycolata. They are responsible for the uptake of hydrophilic compounds across the mycolic acid layer. Cell wall channels of different mycolata have been characterized in recent years including Mycobacterium chelonae and Mycobacterium bovis [15,16], Nocardia glutamicum, farcinica [17,18] Corynebacterium Corynebacterium amycolatum [19-23], diphtheriae, Rhodococcus Corynebacterium equi [24] and Rhodococcus erythropolis [25]. Relatively little is known about the cell envelope of D. maris beyond the presence of arabinoglactan and relatively short mycolic acids with 34-38 carbon atoms and an apparently truncated lipoglycan [26]. In this study, we identified a channel from the cell wall of D. maris. A protein responsible for channel formation was identified with a molecular mass of about 120 kDa, which may be formed from a homooligomer of smaller polypeptides similar to the situation in other members of mycolata, where the cell wall channels are frequently formed by oligomers [18-21] According to results of the lipid bilayer conductance measurements, the cell wall channel of D. maris is wide, water filled and has a high preference for the passage of cations. |D i e t z i a m a r i s m a n u s c r i p t 3 Results and Discussion Isolation and purification of the pore-forming protein from whole D. maris cells Treatment of whole cells of representatives of the mycolata with different detergent containing buffers has been shown to provide an efficient and simple way to isolate poreforming proteins from the cell wall [17]. The use of a number of different detergents allows the removal of the more soluble cell wall components to obtain large amounts of the porin. Here we used a similar method for isolation of the protein responsible for pore-forming activity in D. maris. The cell wall proteins were extracted with different detergents such as LDAO or Genapol X-80 and the supernatants were inspected for pore-forming activity. Addition of the extracts to lipid bilayer membranes resulted in a very fast reconstitution of channels and the highest pore-forming activity was present in the final supernatant containing 1% Genapol, 10mM Tris-HCl, pH 8. SDS-PAGE of the corresponding supernatant demonstrated that it contained a predominant protein band with an apparent molecular mass of 120 kDa and a few other bands. Identification and further purification of the pore-forming protein were achieved by excision of different molecular mass bands from tricine-containing preparative SDS-PAGE gels and their extraction with 1% Genapol, 10mM Tris-HCl, pH 8 overnight at 4°C. The highest pore-forming activity was observed for the 120 kDa band (Fig. 1). To confirm whether the 120 kDa protein forms an oligomer, the protein was boiled at 100ºC for 10 min in sample buffer containing 8 M urea. SDS-PAGE of resulting sample showed that the 120 kDa band was composed of subunits with a molecular mass of about 35 kDa (data not shown). Interaction of the 120 kDa protein from the cell wall of D. maris with lipid bilayer membranes We performed lipid bilayer measurements to study the interaction of the 120 kDa cell wall protein with artificial membranes. Membranes were formed from a 1% (w/v) solution of diphytanoyl phosphatidylcholine dissolved in n-decane. Increase of conductance was not sudden but was observed as a function of time after the addition of the porin to the aqueous phases on one or both sides of membranes in the black lipid state. Alternatively the porin could be added to the aqueous phase prior to membrane formation but in this case no defined sidedness was possible. When different molecular mass regions were excised from the same SDS-PAGE, highest pore-forming activity was always observed for the 120 kDa band. Addition of the pure 120 kDa protein in a very low concentration to the aqueous phase bathing black lipid membranes resulted in a very strong increase of the conductance. Nevertheless, we also observed a small pore-forming activity smeared across the molecular mass region between about 35 and 120 kDa. This result suggested that the 120 kDa band could be an oligomer of smaller |D i e t z i a m a r i s m a n u s c r i p t 4 polypeptides. Control experiments with the detergent Genapol at the same concentrations as with the protein confirmed that the membrane activity was caused by the presence of the cell wall porin and not by the detergent. Single-channel analysis In single-channel experiments, we observed a considerable increase of the specific conductance of the lipid bilayers in the presence of the purified cell wall porin. The addition of lower concentrations (10 ng/ml) of the cell wall porin to lipid bilayers made of diphytanoyl phosphatidylcholine/n-decane allowed the resolution of stepwise conductance increases. Fig. 2A shows a single-channel recording of the D. maris 120 kDa porin activity, 5 min after the addition of the protein to a lipid bilayer membrane. Each step indicated the incorporation of one channel forming unit into the membrane. All the steps were directed upwards, which demonstrated that the channels were always in the open state and had a long lifetime. Fig. 2B shows a histogram of all conductance steps observed in reconstitution experiments with the 120 kDa protein in 1 M KCl and a voltage of 20 mV. The most frequent value for the single-channel conductance of the 120 kDa protein was 5.75 nS. The single-channel conductance of the pore-forming protein from D. maris was also studied as a function of different KCl concentrations (see Table 1). The results clearly demonstrated that the conductance was not a linear function of the KCl concentration in the aqueous phase suggesting that the channel contained either a binding site for cations or anions or that the channel contains point charges. Single-channel experiments were also performed with salts other than KCl to obtain information on the size and selectivity of the channels formed by PorADM. The results are also summarized in Table 1. The conductance sequence of the different salts within the channel was KCl ≈ KCH3COO > LiCl. This means that the influence of cations of different size and mobility on the conductance was quite substantial suggesting cation-selectivity of the PorADm channel (see Table 1). Selectivity measurements Further information about the structure of the 120 kDa porin of D. maris was obtained from zero-current membrane potential measurements in the presence of salt gradients. Fivefold salt gradients (100 mM versus 500 mM) were established across lipid bilayer membranes in which about 100 to 1000 PorADm channels were reconstituted. This gradient resulted in an asymmetry potential of 20.7 (±2.4) mV at the more dilute side of the membrane (mean of 3 measurements). This result indicated preferential movement of potassium ions over chloride through the channel at nearly neutral pH. The zero-current membrane potentials were analyzed using the Goldman-Hodgkin-Katz equation [27]. The ratio of the potassium |D i e t z i a m a r i s m a n u s c r i p t 5 permeability, PK, divided by the chloride permeability, PCl, was about 6.7 (mean of 3 measurements), indicating a relatively high cation selectivity of the channel formed by PorADm. This result was confirmed by measurements with LiCl and potassium acetate where we observed, under the same conditions as for KCl, asymmetry potentials of 19.7 and 24.7 mV at the more dilute side, respectively for fivefold salt gradients (see Table 2). The corresponding ratios of cation permeability Pcation divided by anion permeability Panion were 4.6 for LiCl and 7.3 for potassium acetate. This suggested that size and mobility of cations and anions influenced the cation selectivity of PorADm, which is in agreement to the situation observed previously for wide water-filled cell wall channels of Gram-positive bacteria, where size and hydration shell of the ions influence selectivity [17,19,28,29] The cell wall channel of D. maris is cation selective Similar to channel formed by other cell wall porins such as those from M. smegmatis [30], M. chelonae [31] and N. farcinica [32], the cell wall channel of D. maris is cation selective. This cation selectivity is based on the presence of point charges at the channel mouth because the single channel conductance was dependent on the square root of the cation concentration as the data of Table 1 clearly indicated [33]. A best fit of the concentration dependence of the single channel conductance given in Table 1 was obtained by using the formula of Nelson and McQuarrie [34] and assuming that 2.4 negatively charged groups (q = -3.84 x10-19 As) are located at the pore mouth and that its radius is approximately 1.1 nm. The results of Fig. 3 show that a reasonable fit of the experimental data is achieved for these parameters. Fig. 3 also demonstrates that the influence of the surface charges is rather small at high ionic strength. When the single-channel conductance is corrected for the presence of point charges at the channel mouth, it is a linear function of the aqueous salt concentration (dashed line in Fig. 3). The negative potential at the mouth of the channel has important implications on the function of the cell wall channel of D. maris. At a concentration of 100 mM KCl or NaCl, the potential is approximately -39.7 mV at the channel mouth (as calculated from the Nelson and McQuarrie formula) [34]. This means that the concentration of monovalent cations is increased there to 470 mM (bulk concentration 100 mM; calculated according to c0 c exp( F /( R T )) , while the concentration of monovalent anions is decreased to 21 mM (bulk concentration 100 mM; calculated according to c0 c exp( F /( R T )) . This means that under physiological conditions the channel conducts cations approximately 20times more than anions of the same aqueous mobility without being really selective for cations due to the presence of a selectivity filter for cations. Similar considerations apply to the discussion of the zero-current membrane potentials, i.e. only part of the full bulk aqueous |D i e t z i a m a r i s m a n u s c r i p t 6 gradient drops across the channel. It is noteworthy that also other cell wall porins form channels that are influenced by point charges [17,21,30,31]. The channel formed by the 120 kDa protein is voltage dependent in an asymmetric manner In single-channel recordings the PorADm channel exhibited some flickering at higher voltages, i.e. it showed rapid transitions between open and closed configurations. This could be caused by its voltage-dependent closing. To study this effect in more detail we increased in multi-channel experiments the voltage across the membranes. PorADm was added at a concentration of 100 ng/ml to one side of a black diphytanoyl phosphatidylcholine/n-decane membrane (the trans-side) and the conductance increase was followed for about 30 minutes. After this time the reconstitution rate of PorADm decreased significantly and we applied different negative and positive potentials (with respect to the cis-side, the side opposite to the addition of PorADm) to the membrane, starting from ±120 mV. Then we repeated the experiment with ±20, ±30, ±40 up to ±100 mV. The membrane current started to decrease when the voltage at the cis-side exceeded +20 mV. For negative potentials at the cis-side the current was voltage-independent up to -60 mV. Only for voltages more negative than -70 mV was a decrease was observed. The data of the voltage-dependence experiments were analyzed in the following way: the membrane conductance (G) as a function of voltage, Vm, was measured when the opening and closing of channels reached an equilibrium, i.e. after the exponential decay of the membrane current following the voltage step Vm. G was divided by the initial value of the conductance (G0, which was a linear function of the voltage) obtained immediately after the onset of the voltage. The data in Figure 4 correspond to the asymmetric voltage-dependence of PorADm (mean of three membranes) when the protein was added to the trans-side. This result indicated full asymmetric insertion of PorADm into the membranes when it was added to only one side. The addition of the protein to both sides of the membrane resulted in a symmetric response to the applied voltage (see Figure 4). Estimation of the diameter of the cell wall porin of D. maris Experiments with non-electrolytes (NEs) can be used to provide an estimation of the effective pore diameter of a channel [35-41]. This method was also applied to the conductance of the PorADm channel. The 1 M KCl solution was supplemented for these experiments with 20% (w/v) NEs with different molecular masses ranging from 62 Da (ethylene glycol) to 3 kDa (PEG 3000). This means that the NEs had molecular radii between 0.26 nm to 1.44 nm (see Table 3). The results of single-channel conductance measurements with these NEs suggested that large NEs with hydrodynamic radii between 1.22 and 1.44 nm (PEG 2000 and PEG 3000) did not enter the PorADm channel and showed only little or no effect on its |D i e t z i a m a r i s m a n u s c r i p t 7 conductance. However, in the presence of small NEs with hydrodynamic radii up to 0.80 nm, the single-channel conductance of PorADm decreased approximately proportional to that of the bulk aqueous conductivity (Table 3). Histograms of four representative NEs measurements together with the recordings of single-channel traces are illustrated in Figure 5. For PEG 1000, (hydrodynamic radius 0.94 nm) the single-channel conductance decreased but notably much less than that of the specific conductivity of the aqueous bulk solution (see Table 3 and Fig. 5). These data indicated only partial filling of the PorADm channel with the corresponding NE [35] suggesting that non-electrolytes with a much larger hydrodynamic radius than 0.94 nm were not able to enter the cell wall channel, thus showing no reduction in the pore conductance. In contrast, non-electrolytes with a hydrodynamic radius of 0.8 nm or smaller could enter and presumably also pass through the channel (Table 3). To characterize the pore size of PorADm in more detail, its conductance dependent on the different NEs was plotted as a function of their molecular masses and hydrodynamic radii following established procedures [42]. The ratios of the single-channel conductance in the presence of NEs to that in the absence of NEs as a function of the molecular mass and the NEs radii are shown in Fig.6. The obtained results suggested that NEs with a mean molecular mass (Mr) of ≤ 600 g/mol and a hydrodynamic radius (r) ≤ 0.8 nm enter fully the channel whereas NEs with a Mr ≥ 2000 g/mol and r ≥ 1.22 nm cannot enter the PorADm channel. The results of these measurements suggested that the entrance diameter of PorADm is around 2.1 nm. Comparison of the cell wall channel properties of D. maris and other mycolata The cell envelope of D. maris and members of the closely related genera Corynebacterium, Mycobacterium, Nocardia and Rhodococcus are similar in the composition and organization of the cell wall, with an asymmetric outer lipid layer defined by the presence of mycolic acids [10,11]. Therefore, it is expected that the porins in the cell wall of these bacteria share some common properties (Table 4). These include that they are typically composed of small molecular mass subunits and form large and water-filled channels. These common properties seem to be a characteristic for the cell wall porins of mycolata. However, some exhibit cation selectivity (as in D. maris) whereas others are anion selective, although it is likely that each organism will possess channels with both types of selectivity in order to facilitate uptake of the full range of hydrophilic solutes. More interestingly, some porins appear to work as hetero-oligomers [47,48] whereas others form likely homo-oligomers [49] . Materials and Methods |D i e t z i a m a r i s m a n u s c r i p t 8 Bacterial strain and growth conditions D. maris DSM 43672T cells were grown in GYE Glucose-Yeast Extract medium (10 g Glucose and 10 g Yeast Extract per L distilled water), pH 7.2 at 28 ºC with shaking. Cells were checked for purity and harvested by centrifugation at early stationary phase. The cells were washed twice with sterile distilled water and lyophilized. Isolation and purification of the channel-forming protein from the cell wall For purification of the cell wall porin, we used the same method used for the extraction of the cell wall channel of N. farcinica [17]. The method is based on the use of different detergents to remove most of the soluble cell wall molecules. In the first two steps (I and II), the lyophilized cells were washed in 10mM Tris-HCl, pH 8.0 followed by centrifugation at 4000 rpm in an Eppendorf 5810R centrifuge (rotor A-4-81) for 10 min. The pellet was then treated with two different washing steps (steps III and IV) using 0.2 % SDS, 1% Genapol and 10 mM EDTA followed by centrifugation (11,000 rpm in an Eppendorf 5415R microcentrifuge, 4°C) the final pellet was homogenized in 1% Genapol and 10 mM Tris-HCl, pH 8 for 20 h at 50ºC under agitation. Pore-forming activity was present in the resulting pellet and in its supernatant. SDS-PAGE Analytical and preparative SDS-PAGE was performed according to Laemmli [50]. Because of the low resolution of this gel system for observation of low molecular mass proteins, tricine containing gels were used as described by Schägger [51]. The gels were stained with Coomassie brilliant blue, Colloidal Coomassie blue [52] or with silver stain [53]. Lipid bilayer experiments The methods used for the lipid bilayer measurements have been described previously in detail [54]. A Teflon chamber was divided into two compartments connected by a small circular hole with a surface area of about 0.4 mm2. Black lipid bilayer membranes were obtained by painting a solution of 1% (w/v) diphytanoyl phosphatidylcholine in n-decane onto the hole. All salts were analytical grade and the temperature was maintained at 20°C during all experiments. Zero current membrane potential measurements were obtained by establishing a salt gradient across membranes containing about 100 cell wall porins as described elsewhere [27]. Estimation of the channel diameter using non-electrolytes (NEs) The method used was as described by Krasilnikov et al. in 1998 [35]. Solutions containing 1M KCl were supplemented with 20% (w/v) of an appropriate NE. Single-channel |D i e t z i a m a r i s m a n u s c r i p t 9 measurements were carried out using different NE with increasing hydrodynamic radius. The conductance in the presence of the NEs was calculated from at least 100 single events. Acknowledgements References [1] Nesterenko OA, Nogina TM, Kasumova SA, Kvasnikov EI & Batrakov SG (1982) Rhodococcus luteus nom. nov. and Rhodococcus maris nom. Nov. Int J Syst Bacteriol 32, 1-14. [2] Keorner RJ, Goodfellow M & Jones AL (2009) The genus Dietzia: a new home for some known and emerging opportunist pathogens. FEMS Immunol Med Microbiol 55, 296-305. [3] Zhi XY, Li WJ & Stackebrandt E (2009) An update of the structure and 16S rRNA gene sequence-based definition of higher ranks of the class Actinobacteria, with the proposal of two new suborders and four new families and emended descriptions of the existing higher taxa. Int J Syst Evol Microbiol 59, 589-608. [4] Rainey FA, Klatte S, Kroppenstedt RM & Stackebrandt E (1995) Dietzia, a New Genus Including Dietzia maris comb. nov. Formerly Rhodococcus maris. Int J Syst Bacteriol 45, 32-36. [5] Schuppler M, Wagner M, Schön G & Göbel UB (1998) In situ identification of nocardioform actinomycetes in activated sludge using fluorescent rRNA-targeted oligonucleotide Probes. Microbiology 144, 249-259. [6] Lima TMS, Fonseca AF, Leao BA, Mounteer AH, Totola MR & Borges AC (2011) Oil Recovery from Fuel Oil Storage Tank Sludge Using Biosurfactants. J Bioremed Biodegrad 2, 125. doi:10.4172/2155-6199.1000125.x [7] Goswami G, Chakraborty S, Chaudhuri S & Dutta D (2012) Optimization of process parameters by response surface methodology and kinetic modeling for batch production of canthaxanthin by Dietzia maris NIT-D (accession number: HM151403). Bioprocess Biosyst Eng 35,1375–1388. [8] Niwa H, Lasker BA, Hinrikson HP, Franzen CG, Steigerwalt AG, Whitney AM & Brown JM (2012) Characterization of human clinical isolates of Dietzia species previously misidentified as Rhodococcus equi. Eur J Clin Microbiol Infect Dis 31, 811–820. [9] Nishiuchi Y, Baba T & Yano I (2000) Mycolic acids from Rhodococcus Gordonia and Dietzia. J Microbiol Methods 40, 1-9. [10] Sutcliffe IC, Brown A & Dover L (2010) The rhodococcal cell envelope: composition, organisation and biosynthesis. In Biology of Rhodococcus (Alvarez HM, eds), pp. 2971. Springer Berlin Heidelberg. [11] Brennan P & Nikaido H (1995) The envelope of Mycobacteria. Annu Rev Biochem 64, 29-63. |D i e t z i a m a r i s m a n u s c r i p t 10 [12] Jarlieri V & Nikaido H (1990) Permeability Barrier to Hydrophilic Solutes in Mycobacterium chelonei. J Bacteriol 172, 1418-1423. [13] Gordon HJN & Kajioka R (1977) Permeability barrier to rifampin in mycobacteria. Antimicrob. Agents Chemother 11, 773-779. [14] David HL, Rastogi N, Clavel-Seres S, Clement F & Thorel MF (1987) Structure of the cell envelope of Mycobacterium avium. Zentralbl Bakteriol Mikrobiol Hyg Ser A 264, 49-66. [15] Trias J, Jarlier V & Benz R (1992) Porins in the cell wall of mycobacteria. Science 258, 1479–1481. [16] Lichtinger T, Heym B, Maier E, Eichner H, Cole ST & Benz R (1999) Evidence for a small anion-selective channel in the cell wall of Mycobacterium bovis BCG besides a wide cation-selective pore. FEBS Lett 454, 349-355. [17] Rieß FG, Lichtinger T, Cseh R, Yassin AF, Schaal KP & Benz R (1998) The cell wall channel of Nocardia farcinica: biochemical identification of the channel-forming protein and biophysical characterization of the channel properties. Mol Microbiol 29,139-150. [18] Kläckta C, Knörzer P, Rieß F & Benz R (2011) Hetero-oligomeric cell wall channels (porins) of Nocardia farcinica. Biochim et Biophys Acta 1808, 1601–1610 [19] Schiffler B, Barth E, Daffé M & Benz R (2007) Corynebacterium diphtheriae: Identification and characterization of a channel-forming protein in the cell wall. J Bacteriol 189, 7709-7719. [20] Niederweis M, Maier E, Lichtinger T, Benz R & Krämer R (1995) Identification of channel-forming activity in the cell wall of Corynebacterium glutamicum. J Bacteriol 177, 5716-5718. [21] Lichtinger T, Burkovski A, Niederweis M, Krämer R & Benz R (1998) Biochemical and biophysical characterization of the cell wall channel of Corynebacterium glutamicum: the channel is formed by a low molecular mass subunit. Biochemistry 37, 15024– 15032. [22] Dörner U, Schiffler B, Lanéelle MA, Daffé M & Benz R (2009) Identification of a cellwall channel in the corynemycolic acid-free Gram-positive bacterium Corynebacterium amycolatum. Int Microbiol 12, 29-38. |D i e t z i a m a r i s m a n u s c r i p t 11 [23] Soltan Mohammadi N, Mafakheri S, Abdali N, Barcena-Uribarri I, Tauch A & Benz R (2013) Identification and characterization of the channel-forming protein in the cell wall of Corynebacterium amycolatum. Biochim Biophys Acta 1828, 2574–2582. [24] Rieß FG, Elflein M, Benk M, Schiffler B, Benz R, Garton N& Sutcliffe I (2003) The Cell Wall of the Pathogenic Bacterium Rhodococcus equi Contains Two Channel-Forming Proteins with Different Properties. J Bacteriol 185, 952–2960. [25] Lichtinger T, Reiss G & Benz R (2000) Biochemical identification and biophysical characterization of a channel-forming protein from Rhodococcus erythropolis. J Bacteriol 182, 764-770. [26] Sutcliffe IC (2000) Characterisation of a lipomannan lipoglycan from the mycolic acid containing actinomycete Dietzia maris. Antonie Van Leeuwenhoek. 78:195-201. [27] Benz R, Janko K & Lauger P (1979) Ionic selectivity of pores formed by the matrix protein (porin) of Escherichia coli. Biochim Biophys Acta 551, 238-47. [28] Rieß FG, Dörner U, Schiffler B & Benz R (2001) Study of the properties of a channelforming protein of the cell wall of the gram-positive bacterium Mycobacterium phlei. J Membrane Biol 182, 147-157. [29] Hunten P, Schiffler B, Lottspeich F & Benz R (2005) PorH, a new channel-forming protein present in the cell wall of Corynebacterium efficiens and Corynebacterium callunae. Microbiology 151, 2429-2438. [30] Trias J & Benz R (1994) Permeability of the cell wall of Mycobacterium smegmatis. Mol Microbiol 14, 283-290. [31] Trias J & Benz R (1993) Characterization of the channel formed by the mycobacterial porin in lipid bilayer membranes. Demonstration of voltage gating and of negative point charges at the channel mouth. J Biol Chem 268, 6234-6240. [32] Riess FG, Lichtinger T, Cseh R, Yassin AF, Schaal KP & Benz R (1998) The cell wall porin of Nocardia farcinica: biochemical identification of the channel-forming protein and biophysical characterization of the channel properties. Mol Microbiol 29, 139-150 [33] Maier E, Reinhard N & Benz R (1996) Channel-forming activity and channel size of the RTX toxins ApxI, ApxII, and ApxIII of Actinobacillus pleuropneumoniae. Infect Immun 64, 4415-4423. [34] Nelson AP & McQuarrie DA (1975) The effect of discrete charges on the electrical properties of a membrane. J Theor Biol 55, 13-27 [35] Krasilnikov OV, Da Cruz JB, Yuldasheva LN, Varanda WA & Nogueira RA (1998) A novel approach to study the geometry of the water lumen of ion channels: colicin Ia channels in planar lipid bilayers. J Membr Biol 161, 83-92. |D i e t z i a m a r i s m a n u s c r i p t 12 [36] Rostovtseva TK, Nestorovich EM & Bezrukov SM (2002) Partitioning of differently sized poly(ethylene glycol)s into OmpF porin. Biophys J 82, 160-169. [37] Berestovsky GN, Ternovsky VI & Kataev AA (2001) Through pore diameter in the cell wall of Chara corallina. J Exp Bot 52, 1173-1177. [38] Ternovsky YI, Okada Y & Sabirov RZ (2004) Sizing the pore of the volume-sensitive anion channel by differential polymer partitioning. FEBS Lett 576, 433-436. [39] Vodyanoy I & Bezrukov SM (1992) Sizing of an ion pore by access resistance measurements. Biophys J, 62, 10-11. [40] Sabirov RZ, Krasilnikov OV, Ternovsky VI & Merzliak PG (1993) Relation between ionic channel conductance and conductivity of media containing different nonelectrolytes. A novel method of pore size determination. Gen Physiol Biophys 12, 95-111. [41] Krasilnikov OV, Sabirov RZ, Ternovsky VI, Merzliak PG & Muratkhodjaev JN (1992) A simple method for the determination of the pore radius of ion channels in planar lipid bilayer membranes. FEMS Microbiol Immunol 5, 93-100. [42] Krasilnikov OV (2002) Sizing channels with neutral polymers. In Structure and Dynamics of Confined Polymers (Kasianowicz J, Kellermayer MZ and Deamer D eds), pp. 97-115. NATO Science Series, Springer Netherlands. [43] Rieß FG, Lichtinger T, Yassin AF, Schaal KP & Benz R (1999) The cell wall porin of the gram-positive bacterium Nocardia asteroides forms cation-selective channels that exhibit asymmetric voltage-dependence. Arch Microbiol 171, 173-182. [44] Rieß FG & Benz R (2000) Discovery of a novel channel-forming protein in the cell wall of the non-pathogenic Nocardia corynebacteroides. Biochim Biophys Acta 1509, 485495. [45] Niederweis M, Ehrt S, Heinz C, Klöcker U, Karosi S, Swiderek KM, Riley LW & Benz R (1999) Cloning of the mspA gene encoding a porin from Mycobacterium smegmatis. Mol Microbiol 33, 933-945. [46] Trias J and Benz R (1994) Permeability of the cell wall of Mycobacterium smegmatis. Mol Microbiol 14, 283-290. [47] Barth E, Barcelo MA, Klackta C & Benz R (2010) Reconstitution experiments and gene deletions reveal the existence of two-component major cell wall channels in the genus Corynebacterium. J Bacteriol 192, 786-800. |D i e t z i a m a r i s m a n u s c r i p t 13 [48] Klackta C, Knorzer P, Riess F & Benz R (2011) Hetero-oligomeric cell wall channels (porins) of Nocardia farcinica. Biochim Biophys Acta 1808, 1601-1610. [49] Abdali N, Barth E, Norouzy A, Schulz R, Nau WM, Kleinekathöfer U, Tauch A & Benz R (2013) Corynebacterium jeikeium jk0268 Constitutes for the 40 Amino Acid Long PorACj, Which Forms a Homooligomeric and Anion-Selective Cell Wall Channel. PLoS One 8, 1-17. [50] Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680-685. [51] Schägger H (2006) Tricine–SDS-PAGE. Nature protocols 1, 16-22. [52] Erhardt W, Neuhoff V, Arnold N & Taube D (1988) Improved staining of proteins in polyacrylamide gels including isoelectric focusing gels with clear background at nanogram sensitivity using CBB G-250 and R-250. Electrophoresis 9, 255-262. [53] Gross HJ, Baier H & Blum H (1987) Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis 8, 93-99. [54] Benz R, Janko K, Boos W & Laüger P (1978) Formation of large, ion-permeable membrane channels by the matrix protein (porin) of Escherichia coli. Biochim Biophys Acta 511, 305-319. |D i e t z i a m a r i s m a n u s c r i p t 14 Tables Table 1. Average single-channel conductance of D. maris pore-forming protein in different electrolyte solutions. Electrolyte Concentration (M) G (nS) KCl 0.01 0.5 ± 0.1 0.03 0.75 ± 0.2 0.1 1.5 ± 0.5 0.3 2.5 ± 0.5 1 5.75 ± 0.5 3 12.5 ± 2.5 LiCl 1 2.5 ± 0.5 KCH3COO (pH 7) 1 4 ± 0.5 The membranes were formed by diphytanoyl phosphatidylcholine/ in n-decane. The singlechannel conductance was measured at 20mV applied voltage and T = 20°C. The average single-channel conductance, G (± SD), was calculated from at least 100 single events. |D i e t z i a m a r i s m a n u s c r i p t 15 Table 2. Zero-current membrane potentials, Vm, of PC/n-decane membranes in the presence of PorA of D. maris measured for a 5-fold gradient of different saltsa. Electrolyte Permeability ratios Vm (mV) Pcation/Panion KCl 6.7 20.7 ± 2.4 LiCl 4.6 19.7 ± 1.7 KCH3COO (pH 7) 7.3 24.7 ± 2.3 aVm is defined as the difference between the potential at the dilute side minus the potential at the concentrated side. The aqueous salt solutions were used unbuffered and had a pH of 6, if not indicated otherwise; T = 20°C. The permeability ratio Pcation/Panion was calculated using the Goldman-Hodgkin-Katz equation [27] as the mean of at least 3 individual experiments. |D i e t z i a m a r i s m a n u s c r i p t 16 Table 3. Estimation of the diameter of the cell wall porin of D. maris. Mr (g/mol) r (nm) G (nS) G(+NE)/(G-NE) X (mS cm-1) - - 5.75 ± 0.5 1 110.3 Ethylene glycol 62 0.26 3.25± 0.25 0.57 ± 0.09 57.2 Glycerol 92 0.31 2.50± 0.25 0.43 ± 0.08 49.1 Arabinose 150 0.34 2.75± 0.25 0.48 ± 0.09 63.7 Sorbitol 182 0.39 3.00± 0.25 0.52 ± 0.08 57.8 PEG 300 300 0.60 2.75± 0.25 0.48 ± 0.09 45.5 PEG 400 400 0.70 2.75± 0.50 0.48 ± 0.10 46.4 PEG 600 600 0.80 2.75± 0.25 0.48 ± 0.08 54.1 PEG 1000 1000 0.94 4.75± 0.25 0.83 ± 0.10 49.5 PEG 2000 2000 1.22 5.00± 0.25 0.87 ± 0.11 43.6 PEG 3000 3000 1.44 5.00± 0.25 0.87 ± 0.10 48.9 Non-electrolyte None The aqueous phase contained 1 M KCl and 20% (w/v) of different non-electrolytes. The nonelectrolytes were used to determine the diameter of the 120 kDa porin. The molecular masses and the radii (r) of the non-electrolytes are given together with the conductance of the channel (G ± SD) in the corresponding solution and the conductivity of the solution [40]. The membrane voltage was 20 mV; T = 20°C. |D i e t z i a m a r i s m a n u s c r i p t 17 Table 4. Comparison of the properties of cell wall channels of different mycolata. Cell wall porin G (nS) MW (kDa) Selectivity Voltage dependenc e yes Channel diameter (nm) 2.2 Refs. Corynebacterium diphtheriae CdPorA_ATCC 11913 CdPorH_ATCC 11913 Corynebacterium glutamicum CgPorA ATCC 13032 CgPorH ATCC 13032 Corynebacterium amycolatum. Corynebacterium callunae hyp_CcPorA_ATC C 15991 CcPorH_ATCC 15991 Corynebacterium efficiens CefPorH CefPorA Nocardia farcinica NfpA NfpB Nocardia asteroides Nocardia corynebacteroides Mycobacterium bovis Mycobacterium smegmatis Mycobacterium chelonae Mycobacterium phlei Rhodococcus erythropolis Rhodococcus equi PorA PorB Dietzia maris 2.25 66 small selectivity for cations cation selective - 2.2 [20][21] cation selective yes cation selective yes small selectivity for anions yes cation selective yes cation selective yes [19] 4.7 6.8 5.5 2.5 4.7 6.2 3.8 45 3.0±0.4 [22][23] 2.2 [29] 4.8 6 2.3 [29] 3 6 4 87 31 30 84 5.5 134 cation selective yes 100 cation selective anion selective cation selective Yes no yes 3.0 [45][46] 2.7 59 cation selective yes 2.2 [15] 4.5 135 cation selective yes 1.8 to 2.0 [28] 6 67 cation selective yes 2.0 [25] 4 0.3 5.75 67 11 120 cation selective anion selective cation selective Yes No yes 2.0 [24] 0.9 This study 3 4 0.78 |D i e t z i a m a r i s m a n u s c r i p t 1.4-1.6 [17][18] [43] 1.0 [44] [16] 18 Figure legends Figure 1. 10% SDS-PAGE of final supernatant and purified cell wall porin. Lane 1: Molecular mass markers 170 kDa, 130 kDa, 100 kDa, 70 kDa, 55 kDa, 40 kDa, 35 kDa and 25 kDa. Lane 2: The final supernatant. Lane 3: The pure cell wall porin of D. maris was obtained by elution of the 120 kDa band from tricine-containing preparative SDS-PAGE. Figure 2. (A) Single-channel recording of a PC/n-decane membrane in presence of pure 120 kDa protein of D. maris. The aqueous phase contained 1 M KCl, 10 mM Tris-HCl (pH 8.0), the applied membrane potential was 20 mV and the temperature was 20°C. (B) Histogram of the probability P(G) for the occurrence of a given conductivity unit in PC/n-decane membranes in the presence of the 120 kDa protein of D. maris. The most frequent single-channel conductance (G/nS) was 5.75 nS for a total of about 147 singlechannel events. The data were collected from different individual membranes. Aqueous phase contained 1 M KCl, the applied voltage was 20 mV and the temperature was 20 °C. Figure 3. Single-channel conductance of the cell wall channel of D. maris as a function the KCl concentration in the aqueous phase (filled squares). The solid line represents the fit of the single-channel conductance data with the Nelson and McQuarrie (1975) formulas (eqs. (1) to (3) of Benz and Trias, 1993) assuming the presence of negative point charges (2.4 negative charges; q = -3.84 x 10-19 As) at the channel mouth and assuming a channel diameter of 2.2 nm. c, concentration of the KCl solution in M (molar); G, average singlechannel conductance in nS (nano Siemens, 10-9 S). The broken (straight) line shows the single-channel conductance of the cell wall channel that would be expected without point charges. It corresponds to a linear function between channel conductance and bulk aqueous concentration. Figure 4. Voltage dependence of D. maris 120 kDa protein when the porin was added to one or both sides of the membrane. The ratio of the conductance (G) at an applied voltage (V), divided by the initial value of the conductance (G0), immediately after the turning on of the voltage. The aqueous phase contained 1 M KCl and 100 ng/ml porin. The membranes were formed from diphytanoyl phosphatidylcholine /n-decane at a temperature of 20°C. Figure 5. Single-channel recording and histogram of PorADM in the presence of different non-electrolytes. Ethylene glycol and arabinose with hydrodynamic radii of 0.26 and 0.34 nm can get inside the porin showing reduction in the conductance while PEG 1000 and PEG 2000 apparently can not enter the channel. Figure 6. Dependence of the single-channel conductance, G, of PorADm on the molecular masses and radii of the non-electrolytes (NEs). G(+NE)/G(-NE) (± SD) is the ratio of the mean single-channel channel conductance in the presence of NEs divided by that in the absence of NEs (5.75 nS) at pH 6. Molecular masses and radii of the non-electrolytes were taken from Table 1. |D i e t z i a m a r i s m a n u s c r i p t 19 Figures 1 2 3 130 Fig. 1. |D i e t z i a m a r i s m a n u s c r i p t 20 Fig. 7. Fig. 2. |D i e t z i a m a r i s m a n u s c r i p t 21 Fig. 3 |D i e t z i a m a r i s m a n u s c r i p t 22 Fig. 4. |D i e t z i a m a r i s m a n u s c r i p t 23 Arabinose Ethylene Glycole 3 nS 3 nS 5s 5s PEG2000 PEG1000 4 nS 5s 5 nS 5s Fig. 6. |D i e t z i a m a r i s m a n u s c r i p t 24 Fig. 5. |D i e t z i a m a r i s m a n u s c r i p t 25 Fig. 6. |D i e t z i a m a r i s m a n u s c r i p t 26