Biological Molecules Info Pages
advertisement

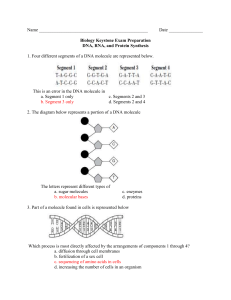
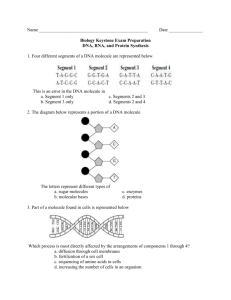
CARBOHYDRATES Examples: sugar, starch, cellulose and glycogen Primary functions: provide and store energy, provide structural support Formed from: Carbon, Hydrogen and Oxygen in a ratio of 1:2:1. Most sugars end with the suffix "-ose". A. Monosaccharides (single molecule sugars) A single molecule sugar is called a monosaccharide (aka simple sugar). Monosaccharides are the monomers of carbohydrates, meaning that they are the smallest molecule into which carbohydrates can be broken down. The prefix “mono” means one. The six carbon monomers below all have the same molecular formula C6H12O6, but have different molecular structures due to a different arrangement of atoms. Three common six carbon monosaccharides are glucose, fructose, and galactose. Examine the structural formulas of these three sugars (Figure 6-1). While most sugars have 6 carbons and are formed in rings, sugars may have 3, 4 or 5 carbons, and may be formed in straight chains or branches as well as rings. (see over) B. Disaccharides (double molecule sugars) Two monosaccharide sugar molecules can join chemically to form a larger carbohydrate molecule called a double sugar, or disaccharide. The prefix “di-“ means two. By chemically joining a Glucose molecule with a Fructose molecule, a double sugar called Sucrose (table sugar) is produced and a molecule of water is released. The process of combining the two monosaccharides is called dehydration synthesis -- making a new molecule out of two while taking a water molecule out. To break this new molecule back into its two original molecules, a molecule of water must be added. This is called hydrolysis -- the adding of a water molecule. ALL organic molecules are put together and broken down by these same processes. Different disaccharide molecules can be made by joining other monosaccharides in different combinations. For example, by chemically joining a Glucose molecule with another Glucose molecule, a double sugar called Maltose is formed. Galactose and Glucose together make Lactose, the sugar found in milk. C. Polysaccharides Just as double sugars were formed from two single sugar molecules, polysaccharides are formed when many single sugars are joined chemically. The prefix “poly-” means many. Starch, Glycogen, and Cellulose are the three most common polysaccharides in biology. They consist of long chains and/or branches of Glucose molecules joined together through dehydration synthesis. Cellulose LIPIDS Examples: Fats, Oils, Waxes, and Steroids. Primary Functions: store energy (fats and oils), provide for chemical communication (steroids), and to provide protection (waxes and oils). Formed of: Carbon, Hydrogen and Oxygen Most lipids are formed with two types of subunits: Glycerol and fatty acids chains. These are called the 'monomers' of lipids, 'mono' meaning "one". These monomers are made of Carbon, Hydrogen and Oxygen (CHO). For most lipids, there will be one Glycerol molecule with 3 fatty acid chains attached. See the diagram below to see how a water molecule is released each time a fatty acid chain is attached to the Glycerol molecule. This is called dehydration synthesis: the making of a new molecule accompanied by the release of a water molecule. To break this fat down, then, a water molecule would need to be added. This is call hydrolysis. These two processes are used every time a new organic molecule is created by combining others, or when an organic molecule is broken down into its monomers. Butyric Acid (one of the fatty acids) Water molecule removed here glycerol When the fatty acid chains consist of only single carbon bonds the lipid is called a saturated fat. If the chains have any carbons double-bonded together, it is called an 'unsaturated fat'. If there are many double-bonded carbons, it is called a 'polyunsaturated fat'. Example of a polyunsaturated fat Lipids are hydrophobic (water hating). One type of lipid, a phospholipid, is used in all cell membranes. This water-hating habit allows the cell membrane to control what goes and in out of the cell. Steroids are formed differently from other lipids; their carbons are usually formed into rings. Hormones are steroidal lipids. These special lipids can pass through the cell membranes. Example of structure of a steroid PROTEINS Examples: muscle tissue, enzymes Formed of: Carbon, Hydrogen, Oxygen, Nitrogen and sometimes Sulfur (CHONS) Functions: support, transport, communication, reaction speeds, cell growth Most end with the suffix "-ase". Proteins are compounds made of small carbon monomers called amino acids. The order and number of amino acids determines which protein is made. There are 20 different amino acids and they all share similar structures. Radical or Variable Group Basic Amino Acid Structure Amino Group (NH2) Carboxyl Group (COOH) C Central carbon atom H 2 examples of Amino Acids Folding of proteins Notice that hooking two amino acids together will release 2 H atoms and 1 oxygen atom (H2O) To form proteins, amino acids are linked into a chain. As each amino acid is added, a water molecule is released This is called dehydration synthesis. When the chain has reached its appropriate length, it is then folded into the unique shape of the protein. A protein can include both fan folded sections and helix (or spring shaped) sections. NUCLEIC ACIDS Nucleic acids: formed of monomers called nucleotides, made up of Carbon, Hydrogen, Nitrogen, Oxygen and Phosphorous atoms (CHNOP). Two basic types of nucleic acids: DNA, and RNA. There are 5 different nucleotides. They share the same basic structure and are known as the 'bases' that make up DNA and RNA (see below). DNA (deoxyribonucleic acid) : a double strand of nucleotide chains in the shape of a double helix. Each of its nucleotides are composed of one of the four bases: Adenine (A), Thymine (T), Guanine (G)and Cytosine (C); a five-carbon sugar called deoxyribose; and a phosphate group. RNA (ribonucleic acid): differs from DNA in that it is single stranded, uses the sugar ribose, and substitutes the base Uracil (U)for Thymine. A single strand of nucleic acid is formed when the phosphate group of a nucleotide attaches to the sugar group of another nucleotide. This then leaves the bases free, as in DNA, to connect to each other with either double or triple hydrogen bonds. (see diagram below) The function of DNA and RNA is to store and transmit genetic information. The DNA that carries the genetic code of the organism is found only in a cell's nucleus; it makes up the cell's chromosomes. (There is another type of DNA; we will learn about that later) There are 3 types of RNA: mRNA (messenger RNA), rRNA (ribosomal RNA) and tRNA (transfer RNA), (We will learn much more about these RNAs later as well.) Basic Structure of a Nucleotide Adenine, Thymine, Guanine, Cytosine or Uracil Deoxyribose in DNA, Ribose in RNA Connecting of Bases to form DNA, a double chain Connecting of nucleotides to form a single chain of nucleic acid 4 Classes of Organic Compounds All Contain Carbon Monomers (M) (Building Blocks) (Building Blocks Macromolecules CHO CHONP Carbohydrates Nucleic Acids M=Sugars M= Nucleotides starch es DNA cellulose RNA glycogen CHONS CHO Lipids M= Fatty Acids and Glycerol Proteins M= Amino Acids fats oils waxes enzymes muscle fibers cytoskeleton steroids