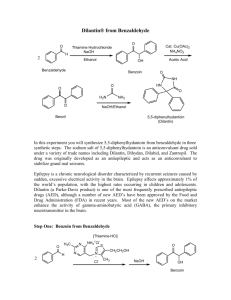
Multistep Synthesis of Benzilic Acid from
Benzaldehyde
Jackson Wyers
March 17, 2013
Texas A&M at Galveston, Galveston, Texas
INTRODUCTION
Benzilic acid is known as an alpha-hydroxy acid or (AHA).
Typically, benzilic acid is an expensive material; however, a three part
synthesis reaction can be conducted in order to convert the rather
inexpensive benzaldehyde. In the overall synthesis, three different
reactions will occur. The first reaction is recognized as the benzoin
condensation reaction and is demonstrated in Equation 1. The
condensation occurs by adding heat to a mixture of benzaldehyde,
thiamine hydrochloride, and sodium hydroxide in an ethanol solution.
Equation 1: Benzoin condensation
this experiment deals with many compounds that have either (O-H)
bonds, (C=O) bonds, or both.
EXPERIMENTAL
A flowchart – Figure 1 – is attached to summarize and outline the
procedures and reagents.
Benzaldehyde to Benzoin. 0.5 grams of thiamine hydrochloride
was dissolved in 0.8mL of water. 5.0mL of 95% ethanol was added
and the solution was then cooled in an ice bath. 1.0mL of 3M sodium
hydroxide was added dropwise while stirring. The pH was tested to
assure it was strongly alkaline. 15.0 mmol of benzaldehyde was then
added to this solution. Parafilm was then used to seal the flask
containing the mixture and was heated on a hot plate for
approximately two hours. Once finished heating, the flask was placed
in a dark drawer and was left undisturbed for one week.
After one week, the mixture was retrieved for separation and
analysis. The inside of the flask was scratched with a glass stir rod to
induce crystallization. Once there was a presence of crystals, the
mixture was cooled in an ice bath and then the benzoin was collected
by vacuum filtration and was washed with both cold water and cold
methanol. Figure 2 can be referred to for visualization on the vacuum
filtration setup. The product was then dried in an oven for several
minutes. Both, IR spectrum and melting point were measured for the
benzoin.
Once the condensation has been executed, the benzoin must undergo
an oxidation reaction in order to yield benzil. This reaction is exhibited
in Equation 2.
Equation 2: Oxidation of benzoin
Figure 2: Vacuum filtration equipment and setup
The final reaction to transpire in the synthesis is the rearrangement of
benzil to give benzilic acid. Equation 3 displays this rearrangement.
Equation 3: Benzilic acid rearrangement
In the synthesis of benzilic acid, cyanide ion is the norm for the
reaction catalyst. Cyanide ion, however, is highly toxic and therefore
Vitamin B1 has been chosen instead. The vitamin is chemically known
as thiamine hydrochloride but will often be notated as thiamine in this
experiment. The purpose of this experiment is then – not only to
conduct a synthesis to yield benzilic acid – but to test the effectiveness
of Vitamin B1 when it serves as a catalyst in place of cyanide ion.
Measuring the melting point of the achieved products
between each step is the ideal way to measure the purity of the
product. To determine purity, the measured melting point is simply
compared to the literature values that are given for the specific
compound. Besides checking the compounds purity, it is of good
practice to run an infrared spectrum on a small sample of the product
to see if the functional groups shown in the IR report match up to the
functional groups of the ideal product. This practice is helpful since
Benzoin to Benzil. To determine the amount of ammonium
nitrate to use in the reaction, the mass of dry benzoin was multiplied
by 0.5. That amount of ammonium nitrate was then added to 3.5mL of
80% acetic acid in a round bottom flask. The benzoin was then added
followed by 0.015 grams of copper (II) acetate. A stir bar was added
and the flask was attached to a condenser which was attached to a
ring stand. A hot plate was placed underneath the flask and the
mixture was heated to start the reaction. When the reaction began,
there was an evolution of a gas. Once the gas evolution had settled,
the mixture was heated to boiling point and then heated under reflux
for one hour. After reflux, the solution was left to cool and while
manually stirring, was added to a small beaker containing crushed ice
using a portion of water to assist the transfer. The mixture was left until
the ice melted and the benzil was then collected by vacuum filtration
and washed several times with cold water. Purification was the next
step of this step. The benzil was recrystallized using 95% ethanol. The
yellow crystals were then washed with cold aqueous 50% ethanol.
Once washed, the solution was then dried and the melting point and IR
spectrum were taken.
Benzil to Benzilic Acid. To determine the amount of 6M
potassium hydroxide to add, the mass of the dry benzil was multiplied
by 2.5. For the amount of 95% ethanol to use, the mass of dry benzil
was multiplied by 3. Benzil was then combined with these two liquids
in a round bottom flask. A stir bar was added and the flask was then
attached to a condenser. Once assembled, the solution was heated
under a gentle reflux for 15 minutes. The mixture was then transferred
to a small beaker containing 10mL of water and continued to slowly
heat on a low temperature (50ºC). Working under the hood, 1.5mL of
concentrated hydrochloric acid was added to a 250mL beaker with a
small portion of crushed ice. 1.5 mL of the potassium benzilate
solution was added to the cold hydrochloric acid mixture and the sides
of the beaker were scratched to assist the formation of crystals. At this
point, the rest of the solution was then added with continuous stirring
and the pH was tested to assure that it was near 2. The solution was
then cooled in and ice bath and the benzilic acid was collected via
vacuum filtration while washing it with cold water. The benzilic acid
was then recrystallized from boiling water. The product was then dried
and weighed. An IR spectrum and the products melting point were
measured. Percent yield calculations were then done for each step of
the synthesis and then for the overall reaction.
Benzoin to Benzil
IR spectrum 3 shows the IR reading for benzil.
RESULTS
The results of the synthesis are split into the three different steps
of the overall reaction.
Benzaldehyde to Benzoin
IR spectrum 1 shows the IR reading for benzaldehyde.
Initial mass of benzoin – 0.51 grams
Mass of benzil recovered – 0.173 grams
Mass of NH4NO3 used – (0.51 grams x 0.5) = 0.255 grams
Melting
Point
93.14ºC
Table 2: The table shows data retrieved for benzil.
Lit. Value
Theoretical
Actual
Percent
Yield
Yield
Yield
95-96ºC
0.505 g
0.173 g
34.26%
0.51g L.R. (benzoin) x (1mol/212.2g benzoin) x (210.2g benzil/1mol) =
.505g = theoretical yield
Percent Yield of Benzil = (0.173g / 0.505g) x 100 = 34.26%
IR spectrum 2 shows the IR reading for benzoin.
Benzil to Benzilic Acid
IR spectrum 4 shows the IR reading for benzilic acid.
An error occurred in this step of the synthesis. Instead of having solid
benzoin form after letting the previously heated benzaldehyde, the
flask simply had a dark orange liquid with no solid. Therefore, 0.51
grams of benzoin was partitioned from the instructor to continue the
synthesis.
Melting
Point
132.27ºC
Table 1: The table shows data retrieved for benzoin
Lit. Value
Theoretical
Actual
Percent
Yield
Yield
Yield
137ºC
1.6 g
0.51 g
31.9%
Percent Yield = (Actual Yield / Theoretical Yield) x 100
Percent Yield of Benzoin = (1.6g / 0.51g) x 100 = 31.9%
Table 3: The table shows data retrieved for benzilic acid
Melting
Lit. Value
Theoretical
Actual
Percent
Point
Yield
Yield
Yield
129.17ºC
150ºC
0.1878 g
0.116 g
61.78%
0.173g of L.R. benzil x (1mol/210.2g benzil) x (228.2g benzilic acid /
1mol) = 0.1878g = theoretical yield
Percent yield of benzilic acid = (0.116g / 0.1878g) x 100 = 61.78%
DISCUSSION
The two major purposes of the experiment were to get benzilic
acid from benzaldehyde and to determine the effectiveness of Vitamin
B1 serving as a catalyst for the reaction. Benzilic acid was successfully
retrieved from the multistep synthesis however, the yields were much
lower than expected. These low yields could imply that the thiamine
hydrochloride is a poor catalyst for the synthesis; yet, thiamine has
been shown by professionals to serve as an effective and efficient
catalyst. Therefore, the low yields are likely due to procedural error.
Had this been a test on a previously untested catalyst, it would be a
good idea to further test and question the effectiveness of the catalyst
since it could be the reason for the low yields. Aside from the low
yields, the overall success of the experiment was above par and the
ideal product was reached.
Judging by the results, the percent yields were an obvious
problem. Out of the three yields, two were in the thirtieth percentile.
However, the final yield of benzilic acid was a good yield at 61.78%.
The percent yield of benzoin was 31.9% which was lower than
intended. The similar problem occurred with the percent yield of benzil
which had a yield of 34.26%. Both of these values fall into the thirtieth
percentile range, which is considerably low when an ideal yield should
have been up into the seventieth percentile or above. It is noteworthy
that the actual yield of 0.116 grams of benzilic acid did not come from
the conducted experiment. In the experiment conducted, there was an
error when the benzilic acid was to be filtered, there was not any solid
recovered. One cause of this error could have been due to the filter
paper that was used. However, this would have only affected a small
portion of the solid so there obviously were other problems. Therefore,
data on amount of benzilic acid had to come from other peer data.
This is the likely explanation as to why the yield is higher and does not
correlate with the yields of benzoin and benzil. It was previously stated
that due to an error in the first step of the experiment, a set amount of
pure benzoin was given to begin the second step. The portions that
were given out varied, and therefore the percent yield of benzoin was
directly determined by how much benzoin the instructor happened to
measure out. The numerous problems were solved as sufficiently as
possible and should not have affected the data any more than the
previously discussed possibilities.
When assessing the IR spectrums, they all appear to correlate
with the structures of the compounds. Benzaldehyde exhibits its (C=O)
stretching at 1702.39 which is in the literature range of 1740-1685.
Benzoin has aromatic peak at 3003.73 which is in the literature range
of 3100-3000. It also shows it (C=O) stretch at 1751.11, again in
range. However, the only problem with the benzoin IR is the depth of
the (O-H) stretch. You should expect to see a large bulge at the 36003200. It does show a stretch at 3413.31, yet it is very shallow. The
likely cause is that not enough of the benzoin sample was placed on
the salt disc when the IR spectrum was run. This would explain why
the (O-H) bulge is shallow and the peaks shallow as well. The
spectrum for benzil looks as it should. There is (C=O) stretching at
1713.04 which falls well within literature values. It also shows its
aromatic peak at 3003.90, which is again ideal. Benzilic acid appears
to have the same problem as seen in benzoin. The peaks are at
accurate frequencies, but the intensities are weak. It shows the (O-H)
stretch at 3379.15 which is ideal, and there is (C=O) stretching at
1678.41 which is in the literature range of 1725-1665. Therefore, all
four IR spectrum readings confirm the presence of the ideal product.
1.
2.
REFERENCES
Lehman, J. W. (2004). Microscale operational organic
chemistry.
(3rd
ed.).
New
Jersey:
Pearson/Prentice Hall.
Figure2.
http://www.chem.wisc.edu/deptfiles/genchem/lab/l
abdocs/modules/vacfilt/pic/trap.gif
Figure 1: Flowchart summary of the overall procedural process.
0.015mol Benzaldehyde
NaOH
Thiamine Hydrochloride (Vit. B1)
Reflux; cool; dark (1 week)
Vacuum filter
Rinse with chilled D.I. H2O
Rinse twice with 1mL chilled methanol
(Solid)
Benzoin – dry in oven 5 mins.
Benzaldehyde
In 10mL RBF,
NaOH
+1/2 mass NH4NO3
Vit. B1
+3.5mL 80% CH3COOH
H2O
+Benzoin
down sink)
+0.015g Cu(CH3COO)2
Heat until gas evolves
Boil, reflux (1Hour)
Air cool. Pour on 8Ml ice.
Vacuum filter
Rinse with chilled H2O
(Filtrate)
(Solid)
Benzil
Recrystallize w/ ethanol
Air cool. Chill on ice.
Cu(CH3COO)2
Vacuum filter
down sink)
Rinse twice w/ 50% ethanol
Save solid. Waste down sink.
(Filtrate)
Benzoin
NH4NO3
(Waste,
(Waste,
Benzil
Weigh. IR. Mp.
In 10mL RBF,
Benzil
+2.5x 6M KOH
+3x Ethanol
Reflux 15 min. after boil
Air cool, add to beaker
Add 10mL DI H2O, heat to dissolve
Pour over 10mL ice/1.5mL HCl(aq) mixture – chill 5 min
Vacuum filter, rinse w/ chilled H2O
(Solid) Benzilic Acid
Oven dry 5 min. weigh. Mp.
Calculate Theoretical Yields
Potassium Benzilate
H2O, Ethanol, KOH
Dispose of waste