2-Aminofurans/JCS
advertisement

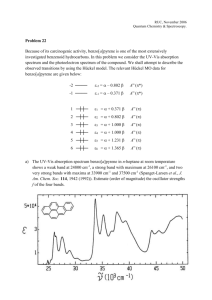
1 A Study of Derivatives Prepared by Oxidative Rearrangement of N2-Arylbenzo[b]furan-2carboximidamides Alžbeta Krutošíková1,* Mária Bencková2, and Christopher A. Ramsden3 1Departmet of Chemistry, Faculty of Natural Sciences, University of St. Cyril and Methodius in Trnava, Slovak Republic; krutosik@ucm.sk 2Department of Organic Chemistry, Slovak University of Technology, SK 812 37 Bratislava, Slovak Republic; benckova@chelin.chtf.stuba.sk 3Department of Chemistry, Keele University, Keele, Staffordshire, UK ST5 5BG; cha33@chem.keele.ac.uk Dedicated to Prof. Dr. Milan Kratochvíl in honour of his 75 birthday _____________________________________________________________________ Introduction 2-Aminofuran Ia and 2-aminobenzofuran IIa are too unstable to be isolated or even trapped as in situ intermediates1. However, simple acetylamino derivatives (e.g. Ib and IIb) are sufficiently stable to be prepared and characterised2-5. Since these compounds cannot be prepared via the unstable amines, access to derivatives that do not contain a stabilising electron-withdrawing group (e.g. NO2, CO2Me) on the furan ring is difficult and information on their chemical properties is extremely limited. O NHR Ia R = H Ib R = COCH3 NHR O IIa R = H IIb R = COCH3 We have recently described a novel (diacetoxyiodo)benzene (DAIB) mediated oxidative rearrangement of C,N-diarylamidines to N-acetylureas4,5. Thermolysis of these ureas results in elimination of an arylisocyanate and formation of an Narylacetamide. The amidines studied included the 2-furyl derivative and the results 2 demonstrated a viable preparative route to the 2-acetylaminofuran Ib from readily available furan-2-carbaldehyde via the 2-furonitrile. Our aim was to compare the behaviour of furan and benzo[b]furan systems, so we have investigated reactions of N2-arylbenzo[b]furan-2-carboximidamides (1a,b) with DAIB. We now report the results of this study. Experimental Melting points were determined using a Kofler hotplate apparatus and are uncorrected. Infrared spectra were recorded on a Philips FTIR PU 9802/25 spectrophotometer with only major absorbances being quoted. Unless otherwise stated IR spectra were measured using KBr pellets (0.5 mg/300 mg KBr). 1H NMR (300 MHz) and 13C NMR (75.43 MHz) spectra were recorded at ambient temperature using a Varian VXR-300 NMR spectrometer with either TMS or HMDS as an internal reference, and were run in deuterated chloroform solution unless otherwise stated. The assignments of the carbon signals were based on the analysis of H,CCOSY spectra. Coupling constants are quoted to the nearest 0.1 Hz. Chemical shifts are quoted in parts per million and the following abbreviations are used: s = singlet; d = doublet; t = triplet; q = quartet; m = multiplet; br = broad. Elemental analyses were determined using either a Perkin-Elmer 240 CHN Elemental Analyser or a Carlo Erba CHNS-OEA 1108-Elemental Analyser. Low resolution mass spectra were recorded on an AEI MS 902-S Mass Spectrometer at 70 eV electron impact ionisation. Separations by column chromatography were carried out using silica gel S (RiedeldeHaen) 0.002-0.063 mm or silica gel (Janssen Chimica) 0.035 - 0.07 mm. All solvents were pre-distilled and dried appropriately prior to use. Concentration and evaporation refer to the removal of volatile materials under reduced pressure on a Büchi Rotovapor. Substances stated to be identical were so with respect to m.p.s, mixed m.p.s and IR spectra. Benzo[b]furan-2-carbonitrile (1) To the mixture of benzo[b]furan-2-carbaldehyde6 (5 g, 34 mmol) pyridine (26 ml), and hydroxylamine hydrochloride (4.75 g, 68 mmol) acetanhydride (20 ml) was added under stirring at 95 °C. The reaction mixture was kept at 85-95 °C for 2h, cooled and poured on ice. The separated precipitate was filtered off and identified as 1 (3.89 g, 80%), pale yellow crystals, m.p. 38 °C (hexane), (ref.7: 36 °C). max/ cm-1 (KBr) 3 2226, 1444, 1180, 750; H 7.25-7.74 (m, 5H, 3H and Harom); (Found: C, 75.38; H, 3.46; N: 7.64. Calc.for C9H5NO: C, 75.52; H, 3.52; N: 7.79%); m/z 143 (M.+). N2-(4-Methylphenyl)benzo[b]furan-2-carboximidamide (2a). To a mixture of benzo[b]furan 2-carbonitrile (4.86 g, 20 mmol) and 4-methylaniline (2.25 g, 20 mmol) in dichloromethane (10 ml) was added powdered aluminium chloride (2.80 g, 20 mmol) in portions with constant swirling. A vigorous exothermic reaction occured and the mixture turned yellow. After evaporation of dichloromethane the thick reaction mixture was added to warm water and alkalised (30% aqueous NaOH) to pH 14. After cooling with ice the solid product was filtered off, air dried, and identified as compound 2a (90%), colourless needles (from toluene), m.p. 188-190 °C (Found: C, 76.74; H, 5.60; N, 11.18. C16H14N2O requires C, 76.78; H, 5.64; N, 11.19%); max/cm-1 3495, 3271, 3112, 1635, 1618, 1589, 1559, 1483, 1449, 1400, 1260, 1234, 1113, 1067; H (d6-DMSO) 2.26 (s, 3H, CH3), 6.42 (br s, 2H, NH), 6.83 (br d, 2H, J 8.1, H-2', H-6'), 7.13 (d, 2H, J 8.1), 7.30 (t, 1H, J 7.9), 7.41 (t, 1H, J 8.2), 7.55 (s, 1H), 7.64 (d, 1H, J 8.2), 7.73 (d, 1H, J 7.9); C (d6-DMSO) 20.62 (CH3), 106.99 (C3), 111.65 (C-7), 121.49 (C-2', C-6'), 122.22 (C-4), 123.55 (C-5), 125.98 (C-6), 127.76 (C-3a), 129.85 (C-3', C-5'), 131.13 (C-4'), 146.62 (C-1'), 146.64 (C-8), 151.23 (C-2), 154.27 (C-7a); m/z 250 (M.+, 100%). N2-Phenylbenzo[b]furan-2-carboximidamide (2b). To a mixture of benzo[b]furan 2carbonitrile (7.15 g, 50 mmol) and aniline (4.65 g, 50 mmol) in dichloromethane (20 ml) was added powdered aluminium chloride (6.65 g, 50 mmol) in portions with constant swirling. A vigorous exothermic reaction occured and the mixture turned orange. After evaporation of dichloromethane the thick mixture was added to warm water and alkalised (30% aqueous NaOH) to pH 14. The solid product was collected, air dried, crystallized and identified as compound 2b (93%), colourless needles (from toluene, 2-methylpentane 1:1), m.p. 181-183 °C (Found: C, 76.19; H, 5.09; N, 11.99. C15H12N2O requires C, 76.25; H, 5.12; N, 11.86%); max/cm-1 3495, 3271, 3112, 1635, 1618, 1589, 1559, 1483, 1449, 1400, 1260, 1250, 1238, 1234, 1186, 1113, 1067, 1007; H 5.01 (br s, 2H, NH), 6.98-7.7 (m, 10H, H-3 and Harom); m/z 236 (M.+). Reactions of benzo[b]furan-2-carboximidamides 2 with (diacetoxyiodo)benzene (DAIB) 4 A solution of amidine 2a (1.25 g, 5 mmol) in toluene (25 ml) was added dropwise to a suspension of DAIB (1.61 g, 5 mmol) in distilled toluene (25 ml) heated in an oil bath maintained at 60 oC and after evaporation the reaction mixture was separated by column chromatography (silica gel, CHCl3 as eluent) to give the following products: N1-Acetyl-N2-(benzo[b]furan-2-yl)-N'-(4-methylphenyl)urea (3a) (16%), pale beige needles (from 2-methylpentane), m.p. 101-103 °C (Found: C, 69.98; H, 5.12; N, 9.09. C18H16N2O3 requires C, 70.12; H, 5.23; N, 9.09%); max/cm-1 (KBr) 3212, 1730, 1686, 1595, 1541, 1454, 1410, 1366, 1314, 1277, 1258, 1238, 1204, 1175, 1109, 1057, 1008; H 2.18 (s, 3H, CH3), 2.31 (s, 3H, CH3), 6.08 (s, 1H, H-3), 7.12 (d, 2H, J 8.4, H-3', H-5'), 7.29 (t, 1H, J 8.1, H-5), 7.37 (t, 1H, J 7.8, H-6), 7.42 (d, 2H, J 8.4, H2', H-6',), 7.51 (d, 1H, J 7.8, H-7), 7.61 (d, 1H, J 8.1, H-4), 10.97 (bs, 1H, NH); C 20.84 (Ph-CH3), 24.99 (COCH3), 105.45 (C-3), 111.71 (C-7), 120.30 (C-2', C-6'), 121.69 (C-4), 123.46 (C-5), 125.51 (C-6), 127.55 (C-3a), 129.54 (C-3', C-5'), 134.15 (C-1'), 134.53 (C-4'), 146.25 (C-2), 150.42 (NH-CO), 153.05 (C-7a), 174.85 (CH3CO); m/z 308 (M.+). 2-(4-Methylphenylamino)-4-(benzo[b]furan-2-yl)benzo[4,5]furo[2,3-d]pyrimidine (4a) (5%), yellow needles (from toluene), m.p. 257-258 °C. (Found: C, 76.64; H, 4.18; N, 10.74. C25H17N3O2 requires C, 76.71; H, 4.38; N, 10.74%); max/cm-1 (KBr) 3432, 3272, 1664, 1611, 1584, 1556, 1522, 1458, 1261, 1196, 1111; H (d6DMSO) 2.30 (s, 3H, CH3), 7.19 (d, 2H, J 8.1, H-3'', H-5''), 7.41 (t, 1H, H-5'), 7.467.58 (m, 3H, H-6, H-7, H-6'), 7.68 (m, 1H, H-8), 7.76 (d, 2H, J 8.1, H-2'', H-6''), 7.85 (s, 1H, H-3'), 7.88 (d, 1H, J 7.7, H-4'), 7.95 (d, 1H J 8.1, H-7'), 8.07 (m, 1H, H-5), 9.98 (br s, 1H, NH); C (d6-DMSO) 20.01 (CH3), 101.88 (C-4a), 109.46 (C-3'), 111.02 (C-8), 111.41 (C-7'), 119.38 (C-2'', C-6''), 120.57 (C-4b), 122.30 (C-4'), 123.16 (C-5), 123.66 (C-5'), 124.14 (C-6), 126.44 (C-6'), 127.02 (C-7), 127.16 (C3'a), 128.62 (C-3'', C-5''), 130.77 (C-4''), 137.17 (C-1''), 149.69 (C-4), 152.59 (C-8a), 153.15 (C-2'), 155.01 (C-7'a), 157.76 (C-9a), 170.46 (C-2); m/z 391 (M.+). N1-[N-(4-methylphenyl)carbamoyl]-N2-(4-methylphenyl)benzo[b]furan-2carboximidamide (5a) (3%) (from toluene-2-methylpentane 9:1), m.p. 197-199 °C 5 (Found: C, 75.06; H, 5.38; N, 10.94. C24H21N3O2 requires C, 75.18; H, 5.52; N, 10.96%); max/cm-1 (KBr) 3438, 3027, 2919, 1698, 1647, 1630, 1613, 1597, 1541, 1505, 1264, 1234, 1183, 1107; H 2.31 (s, 3H, CH3); 2.41 (s, 3H, CH3); 6.17 (s, 1H, H-3); 6.70 -7.60 (m, 12H, H-4,5,6,7 and Harom); 8.00 (br s, 1H, NH); 11.60 (br s, 1H, NH); m/z 383 (M.+). 2-(Benzo[b]furan-2-yl)-5-methyl-1H-benzimidazole (6a) (R = Me) (6%)(from toluene-2-methylpentane 9:1); m.p. 207-210 °C (Found C, 77.48; H, 4.87; N, 11.28. C16H12N2O requires C, 77.40; H, 4.75; N, 11.28%); H (d6-DMSO) 2.43 (s, 3H, CH3); 7.07 (d, 1H, H-6, J6.7 8.06); 7.34 (t, 1H, H-5'); 7.42 (t, 1H, H-6'); 7.47-7.58 (broad signal, H-4 and H-7); 7.62 (s, 1H, H-3'); 7.70 (d, 1H, H-6, J6.7 8.05); 7.78 (d, 1H, H-4', J4',5' 8.06); 13.04 (br s, 1H, NH); C (d6-DMSO) 21.31 (CH3), 105.87 (C3'), 111.39 (C-7'), 121.98 (C-4'), 123.71 (C-5'), 125.73 (C-6'), 128.01 (C-3'a), 147.42 (C-2'), 154.43 (C-7'a), 118.71, 124.74, 131.30, 132.70, 134.73, 142.00, 142.82 (C-2, C-3a, C-4, C-5, C-6, C-7, C-7a); m/z 248 (M.+). In a similar manner N2-phenylbenzo[b]furan-2-carboximidamide (2b) gave: N1-acetyl-N1-(benzo[b]furan-2-yl)-N2-phenylurea (3b) (14%), pale beige needles (from 2-methylpentane), m.p. 102-105 °C. (Found: C, 69.26; H, 4.66; N, 9.62. C17H14N2O3 requires C, 69.38; H, 4.79; N, 9.52%); max/cm-1 (KBr) 3243, 3200, 3121, 3061, 1736, 1732, 1686, 1593, 1545, 1541, 1493, 1447, 1373, 1316, 1306, 1275, 1030, 1010; H 2.17 (s, 3H, CH3), 6.79 (s, 1H, H-3), 7.09-7.67 (m, 9H, Harom), 11.02 (br s, 1H, NH); m/z 294 (M.+). 2-Phenylamino-4-(benzo[b]furan-2-yl)benzo[4,5]furo[2,3-d]pyrimidine 4b (4%), yellow needles (from DMF), m.p. 277-279 °C (Found: C, 76.42; H, 4.08; N, 11.06. C24H15N3O2 requires C, 76.38; H, 4.01; N, 11.13%); max/cm-1 (KBr) 3345, 3056, 1632, 1605, 1586, 1541, 1526, 1501, 1458, 1447, 1427, 1360, 1262, 1194, 1188, 1113; H (d6-DMSO) 6.98-7.89 (m, 13H, Harom), 8.60-8.71 (m, 1H, H-5), 10.03 (s, 1H, NH); m/z 377 (M.+). N1-(N-Phenylcarbamoyl]-N2-(phenyl)benzo[b]furan-2-carboximidamide (5b) (8%), colourless needles (from toluene-2-methylpentane), m.p. 171-175 oC (Found: C, 6 74.22; H, 4.74; N, 11.88. C23H19N2O3 requires C, 74.35; H, 4.82; N, 11.82%); max/cm-1 (KBr) 3432, 1694, 1642, 1620, 1599, 1561, 1545, 1499, 1269, 1260, 1181, 1115; H (d6-DMSO) 6.87 -7.76 (m, 15H, H-3,4,5,6,7 and Harom); 9.51 (s, 1H, NH); 9.80 (s, 1H, NH); m/z 355 (M.+). Thermolysis of N1-acetyl-N1-(benzo[b]furan-2-yl)-N2-(4-methylphenyl)ureas (3) A mixture of compound 3a or 3b (11 mmol) and freshly distilled aniline (1.05 g, 11 mmol) in toluene (100 ml) was stirred in an oil bath at 110 °C and maintained under reflux (1 h). The mixture was allowed to cool to room temperature and the N, N'diphenylurea or N-phenyl-N'-4-methylphenylurea that separated was collected. Evaporation of the yellow filtrate gave a solid that was purified by column chromatography (silica gel 2-metylpentane-ethyl acetate 2:1 as eluent) and identified as 2-acetylaminobenzo[b]furan (7) (89%) (from toluene-2-methylpentane 9:1), m.p. 129-130 °C (lit.3 129-130 oC) (Found: C, 68.4; H, 5.1; N, 7.8. C10H9NO2: requires C, 68.5; H, 5.1; N, 8.0%); max/cm-1 (KBr) 3272, 3216, 1684, 1674, 1607, 1551, 1456, 1248, 1190; H 2.23 (s, 3H, CH3); 6.75 (s, 1H, H-3), 7.00-7.60 (m, 4H, Harom); 8.41 (br s, 1H, NH). Results and Discussion Amidines are potentionally useful precursors of a variety of molecules of biological interest. There are many methods for preparation of amidines8. We were intrested in that one which is using heterocyclic carbonitriles because their synthesis is very simple via one pot synthesis from corresponding heterocyclic carbaldehydes. Benzo[b]furan-2-carbaldehyde6 was treated with hydroxylamine hydrochloride in the presence of acetanhydride in pyridine giving 1 in very good yield. Amidines 2a,b were synthesised by reaction of carbonitrile 1 with 4-methylaniline or aniline in the presence of aluminium chloride in dichloromethane at room temperature. The formed amidineAlCl3 complex was decomposed by warm water and alkalised. 7 4 R NH2 5 CN R NH 2 6 O 3 3a 7 N H O 7a 1 2a,b 1 DAIB 4 5 3 3a 2 6 7 O 7a aniline NHCOMe 4 3 3a COMe 5 6 O 7a 7 N 2 CONH 7 4´ 1´ 2´ 3a,b 3´ 5´ R 4´ 6´ 7´ R 3´ 4 3 7 7a O 1´O N 5 6 R 2 5 6 NHCONH R N O 8 3´´ N3 4b 4a 7 5a,b 4´´ 2´ 4 2 1 N H 1´´ 2´´ 9 4a,b 4´ 3´ 3 5´ 6´ 7´ R 4 3´a 7´a O 2´ 2 1´ N 5 6 N H 7 1 6a R = in: a = Me, b = H Scheme 1 Both amidines were isolated in very good yields after crystallisation from toluene and 2-methylpentane. The reaction of benzo[b]furan-2-carboximidamides with (diacetoxyiodo)benzene was carried out at 60 °C in toluene and after evaporation the reaction mixture was separated by column chromatography (silicagel chloroform as eluent) to give products 3-6 (Scheme 1). The structure of the compounds was proved by 1H and 13C NMR spectroscopy. The structure of the compound 4a was proved by X-Ray analysis. From reaction mixture of the compounds 2a with DAIB the compound 6a was isolated as well. The assignment of the 13C signals of benzimidazole part of the compound 6a is complicated owing to the prototropy on the benzimidazole ring and the carbon signals have not been assigned unambiguously. The comparison of 13C NMR data with those of benzimidazole and some its derivatives published9 has been used for the carbon assignments. 8 The final step-the preparation of 2-acetylaminobenzo[b]furan (7) was carried out by treatment of the corresponding compounds 3 with one equivalent of aniline in toluene at reflux and isolated by column chromatography. The compound 7 was previously described in ref.7 In conclusion, we can state on the present synthesis and our previous studies of furan and benzo[b]furan ring system that the second one is more stable the first one. We suppose that is a reason that we were able to isolate four reaction products. We noticed a remarkable difference in solubility of both system derivatives. Furthermore we will present more details in our prepared paper10. This study was supported by the Grant Agency of the Slovak Ministry of Education (projects: 1/4207/97, 1/6249/99) and The Royal Society (London). The authors are indebted to Mrs O. Lakatošová, and Dr. M. Dandárová for the measurement of IR and NMR spectra. The excellent assistance of Mrs. J. Lehká is gratefully acknowledged. References 1. Lythgoe D. J., McClenaghan I., Ramsden C. A.: J. Heterocycl. Chem., 1993, 30, 113. 2. Singleton H. M., Edwards, Jr. W. R.: J. Am. Chem. Soc., 1938, 60, 540. 3. Abramenko P. I., Zhiryakov V. G.: Khim. Geterotsikl. Soed., 1977, 1495. 4. Ramsden C. A., Rose H. L: J. Chem. Soc. Perkin Trans.1, 1995, 615. 5. Ramsden C. A., Rose H. L: J. Chem. Soc. Perkin Trans.1, 1997, 2319. 6. Krutošíková A., Kováč J., Dandárová M., Bobáľová M.: Collect. Czech. Chem. Commun. 1982, 47, 3288. 7. Rene L, Royer R.: Bull. Soc. Chim. Fr. 1971, 4329. 8. Gautier J.-A., Miocque M., Farnoux C. C., in: „The Chemistry of Amidines and amidates“, Vol. 1. Ed. S. Patai, Wiley, London 1975. 9. Goljer I., Milata V., Ilavský D.: Magn. Reson. Chem. 1989, 27, 138. 10. Bencková M., Clegg V., Coles S. J., Dandárová M., Hursthouse M. B., Kiss T., Krutošíková A., Liptaj T., Ramsden C. A., prepared for publication in: J. Chem. Soc. Perkin Trans.1