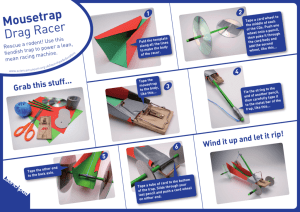
SUPPLEMENTARY DOCUMENTATION FOR THE NMES CD
advertisement

SUPPLEMENTARY DOCUMENTATION FOR THE NMES CD-ROM This file provides additional supporting documentation for this CD-ROM. These documents were originally provided as hard-copy attachments to individual public use tape releases. For the convenience of the user, they are now provided on this file. Additionally, the following documents are of relevance to the user. National Medical Expenditure Survey, Questionnaires and Data Collection Methods for the Household Survey and the Survey of American Indians and Alaska Natives, Methods 2, by W.S.Edwards and M. Berlin, DHHS Publications No. (PHS)89-3450 National Medical Expenditure Survey, Questionnaires and Data Collection Methods for the Medical Provider Survey, Methods 4, by K. Tourangeau and E.P. Ward, AHCPR Pub No. 92-0042 These documents are available from the Agency for Health Care Policy and Research's Publications Clearinghouse at 1-800-358-9295 or from your university's Official Representative to the ICPSR. For reasons of space, the Household Survey Methods 2 does not reproduce every question for every round in full. Information on frequency of provider contacts, type of provider, conditions associated with each contact, and charges and sources of payment was obtained at each round of the Household Survey in separate event booklets. A representative example is provided in Methods 2 for dental visits. Booklets for other provider types are contained in Methods 2 but excluding questions on charges and sources of payment, which were essential identical to those asked for dental visits. The Medical Provider Survey questionnaire is reproduced in full in the Methods 4 document, except for repeat series. A listing of supporting documentation on this file follows. NMES Data Items for the Civilian Noninstitutionalized Population Information on Previously Released NMES Public Use Tapes Variance Estimation Programs for Use with Complex Survey Data National Health Interview Survey Procedure Codes for Use with the NMES Household Survey Data X-Codes for Special Impairments (X00-X99) by Type, Site and Etiology Medicine Names and Codes (Koch, 1980) Generic Drug Names and Codes (Koch, 1980) T Questions from Round 1 of the Household Survey Technical Specifications for Utilization and Expenditure Variables Comparison of Estimates of NMES Health Care Utilization Using NMES Public Use Tapes 13, 14, and 18 Comparison of Estimates of NMES Health Care Expenditures Using NMES Public Use Tapes 13, 14, and 18 The remainder of this file contains these supplementary documents in the order shown above. Users can use the DOS FIND command with the /n/i options to locate specific documents. NMES DATA ITEMS FOR THE CIVILIAN NONINSTITUTIONALIZED POPULATION DATA ITEM RECORD IDENTIFIERS AND LINK VARIABLES Person level Family level by round Family level (annual) ODU level RU level by Rd Event level Medical provider identifiers Insurance provider identifiers Policyholder-level identifiers Links to HIPS private insurance benefit database Minor's parent/guardian identifiers Health insurance eligibility unit NMES TAPE(S) ON WHICH DATA IS AVAILABLE: 9, 10, 13, 14.1-14.5, & 15*, 16*, 18 13, 18 18 9, 10, 13, 14.1-14.5, & 15*, 16*, 18 13, 18 14.1-14.5 9, 14.2, 14.4, 14.5 15*, 16* 15*, 16* 16* 18 by round, annual 18 SURVEY ADMINISTRATION AND ELIGIBILITY STATUS Eligibility status for HS by Rd 13, 18 Interview dates for health status questionnaire and access data 9 Interview dates for core questionnaire by Rd 13 Reference period dates by Rd 13 Respondent identifiers by Rd 13 Round of data collection 14.1-14.5 Response disposition code in HIPS 15*, 16* Interview conducted in English 18 Memory aids used in survey recall 18 SAMPLING WEIGHTS AND VARIANCE ESTIMATION Health status questionnaire/access supplement weight (HSQACCWT) 9 Preliminary Round 1 weight (INCR1PER) 10 Final Round 1 Person-level weight (WGTR1PER) 13, 18 Preliminary Round 4 weight (INCR4PER) 10 Final Round 4 Person-level weight (WGTR4PER) 13, 18 Full-year weight (INCALPER) 10, 13, 14.1-14.5, 18 Policyholder weight (POSTJO2) 15*, 16* Round 1 family-level weight (FAMID1WT) Round 4 family-level weight (FAMID4WT) Full-year family-level weight (ANFMIWT) Full-year annualization factor (ANFACTOR) Round 1 Health Insurance Unit Level weight (HIEU1WT) Round 4 Health Insurance Unit Level weight (HIEU4WT) Full-year Health Insurance Unit Level weight (ANHIEUWT) Strata Primary sampling unit DEMOGRAPHICS Census region - R1 Census region - R4 Census division SMSA status (SMSA/Non-SMSA) 18 18 18 18 18 18 18 9, 10, 13, 14.1-14.5, & 15*, 16*, 18 9, 10, 13, 14.1-14.5, & 15*, 16*, 18 9, 10, 13, 14.1-14.5, & 15*, 16*, 18 15*, 16*, 18 13 13 SMSA/density (SMSA core county/ SMSA non-core/non-SMSA) Age (First age and Last age) Age by RD Race Race/Hispanic ethnicity Hispanic ancestry group Sex Education Native Language Veterans status - R1 Current/past military service Service related disability Veterans status - R4 Vietnam veteran Branch of the armed services FAMILY RELATIONSHIPS Marital status by Rd Parent identifiers Spouse identifiers by Rd Relationship to family reference person by Rd Family reference person identifiers Family size Vital status of parents not in household Age of parents not in household Childcare arrangements for youngest child Minor's parent/guardian identifiers Parents with mental/physical difficulties HEALTH STATUS AND ATTITUDES Height and weight Health practices Health status Check lists of health conditions Check lists of symptoms Immunizations for children 17 and younger Vision and hearing Dental care Health perceptions Social activities ADL/IADL difficulties Continence 18 9, 10, 13, 14.1-14.5, & 15*, 16*, 18 13 9, 10, 13, 14.1-14.5, & 15*, 16*, 18 9, 10, 13, 14.1-14.5, 15*, 16*, 18 13 9, 10, 13, 14.1-14.5, & 15*, 16*, 18 13 9 13 13, 18 13 18 18 18 13 13, 18 13 13 13 13 18 18 18 18 18 9 9 9 9 9 9 9 9 9 9 10 10 Use of special equipment Use of special transportation, etc. for those with at least one ADL/IADL Prior nursing home stays for those with at least one ADL/IADL 1987 admission to a institutional facility Loss of a close friend or relative Close friend or relative institutionalized Persons with developmental disabilities Pregnancy in 1987 Prenatal care ACCESS TO CARE Usual source of care Characteristics of the usual source of care and regular medical providers Reasons for no usual source of care Usual source of dental care Travel and waiting times Barriers to care Medical provider identifier for the usual source of care INCOME AND POVERTY STATUS Total family income Total person income Poverty status Amount of income from 26 sources (e.g., from business, wages, etc) EMPLOYMENT Employment status in week prior to interview by Rd Employment status during the Rd Details about workers laid off or without work by Rd Items for main/last job(s): Wage rate Usual hours worked per week Job start/end dates Size of employer Union membership Industry and occupation codes Other job characteristics Annual weeks worked Employment status as of 12/31/87 Type of employer Link to last main job 10 10 10 18 18 18 18 18 18 9 9 9 9 9, 14.5 9 9, 14.2, 14.4, 14.5 13 13 13 18 13 13, 18 13 13 13 13 13 13 13 13 13 13 13, 18 18 15*, 18 Changed job during 1987 13 HOUSEHOLD SURVEY REPORTED INSURANCE COVERAGE Medicare coverage as of the interview date (as of Dec 31,1987 13 for last round) CHAMPUS/CHAMPVA as of the interview date 13 Medicaid as of the interview date 13 Other public medical assistance as of the interview date 13 Private health insurance as of the interview date 13 Uninsured as of the interview date 13 Sources of private insurance plans by Rd 13 Policy holders of priv. insurance plans by Rd 13 Medicare coverage by month 18 CHAMPUS/CHAMPVA by month 18 Medicaid by month 18 Other public medical assistance by month 18 Private health insurance by month 18 Uninsured by month 18 Difficulty in purchasing insurance 18 HMO coverage as of the interview date by round 18 HMO coverage at anytime during RD 18 ANNUAL Number Number Number Number PERSON-LEVEL DISABILITY MEASURES of bed disability days 13 of work-loss days 13 of school-loss days 13 of cutdown days 13 HOUSING DATA Type of housing unit Mortgage and tax data Purchase price of home Profit or loss from selling home Home located in a retirement community Services available in a retirement community Modifications made to home for handicapped persons TAX DATA Tax filing status Number and relationship of dependents to taxpayer Itemized medical expenses Tax deduction for medical expenses 18 18 18 18 18 18 18 18 18 18 18 ANNUAL PERSON-LEVEL UTILIZATION MEASURES Number of medical provider visits: To a physician 13, To a non-physician provider 13, Telephone calls to physician 13, Telephone calls to a non-physician medical provider 13, Number of hospital outpatient visits: To a physician 13, To a non-physician medical provider 13, Number of emergency room visits 13, Number of emergency room visits resulting in a hospitalization 13, Number of hospital inpatient stays 13, Number of nights spent in a hospital 13, Number of home health services: Provided by physicians 13, Provided by non-physician medical providers 13, Number of prescribed medicine purchases 13, Number of dental visits 13, Number of dental visits involving orthodontia 13, 14.5, 18 14.5, 18 14.5, 18 14.5, 18 14.5, 18 14.5, 18 14.5, 18 14.5, 18 14.4, 18 14.4, 18 14.2, 18 14.2, 18 14.1, 18 14.3, 18 14.3, 18 ANNUAL PERSON-LEVEL EXPENDITURE AND SOURCES OF PAYMENT (SOP) MEASURES Expenditure and SOP for medical provider visits: To a physician 18, 14.5 To a non-physician provider 18, 14.5 Telephone calls to physician 18, 14.5 Telephone calls to a non-physician medical provider 18, 14.5 Expenditure and SOP for hospital outpatient visits: To a physician 18, 14.5 To a non-physician medical provider 18, 14.5 Expenditure and SOP for emergency room visits 18, 14.5 Expenditure and SOP for emergency room visits resulting in a hospitalization 18, 14.5 Expenditure and SOP for hospital inpatient stays 18, 14.4 Expenditure and SOP for nights spent in a hospital 18, 14.4 Expenditure and SOP for home health services: Provided by physicians Provided by non-physician medical providers Expenditure and SOP for prescribed medicine purchases Expenditure and SOP for dental visits Expenditure and SOP for dental visits involving orthodontia Expenditure and SOP for eye glasses/ contact lenses Expenditure and SOP for durable goods (excluding vision wear) Expenditure and SOP for purchases or rental of non durable goods (e.g., bandages, ambulance service, catheters) 18, 14.2 18, 14.2 18, 14.1 18, 14.3 18, 14.3 18, 14.2 18, 14.2 18, 14.2 1987 PRESCRIBED MEDICINE USE AND EXPENDITURE EVENT-LEVEL DATA Types of prescribed medicines 14.1 Household respondent reported medical conditions associated with prescribed medicines obtained 14.1 Types of generic equivalents 14.1 Federal controlled substance status 14.1 Composition status 14.1 Dates first and last taken 14.1 Number of purchases 13, 14.1, 18 Total expense 14.1, 18 Sources of payment from nine sources 1987 HOME HEALTH VISIT AND EXPENDITURE Beginning and ending dates of HH care Type of provider performing HH care Provider's place of work Time of day of visits Length of visits Household respondent reported medical conditions associated with home health care Number of visits by provider type Medical or nursing treatments performed ADL or IADL assistance performed Total expense for visit Nine sources of payment for last visit 1987 OTHER MEDICAL EXPENSES USE AND EXPENDITURE EVENT-LEVEL DATA 14.1, 18 EVENT-LEVEL DATA 14.2 14.2 14.2 14.2 14.2 14.2 13, 14.2, 18 14.2 14.2 14.2, 18 14.2, 18 Type of medical item bought/rented/ used Date medical item first obtained Medical conditions associated with item bought Total expense Sources of payment from nine sources 14.2, 18 14.2 14.2 14.2, 18 14.2, 18 1987 DENTAL VISIT AND EXPENDITURE EVENT-LEVEL DATA Type of dental services provided 14.3 Number of dental visits 13, 14.3, 18 Dental visits due to accidents or injury 14.3 Household respondent reported medical conditions associated with the dental visit 14.3 Total expense 14.3, 18 Sources of payment from nine sources VA facility indicator 14.3, 18 14.3 1987 HOSPITAL INPATIENT STAY AND EXPENDITURE EVENT-LEVEL DATA Dates entered and left hospital 14.4 Number of hospital stays 13, 14.4, 18 Number of nights in hospitals 13, 14.4, 18 Reason for hospital stay 14.4 Household respondent reported medical conditions associated with the hospital stay 14.4 Operations performed 14.4 Procedures performed 14.4 Facility expense and nine associated sources of payment 14.4, 18 Doctor expense and nine associated sources of payment 14.4, 18 Private duty nurse expense 14.4 VA facility indicator 14.4 1987 MEDICAL PROVIDER VISIT AND EXPENDITURE EVENT-LEVEL DATA Date of medical visit 14.5 Number of medical provider visits 13, 14.5 Location of service 14.5 Physician/non-physician provider 14.5, 18 Type of medical provider 14.5 In person/telephone visit 14.5, 18 Medical visits by site 14.5 Reason for the visit 14.5 Household respondent reported medical condition associated with the medical provider visit 14.5 Type of specialist seen during visit Surgical procedures performed during visit Types of tests administered during visit Total expense for visit Source of payment from nine sources 14.5 14.5 14.5 14.5, 18 14.5, 18 1987 HOSPITAL OUTPATIENT DEPARTMENT VISIT AND EXPENDITURE EVENT-LEVEL DATA Date of medical visits 14.5 Number of medical visits 13, 14.5 Type of provider 14.5 Number of zero night hospital stays during 1987 14.5 Reason for the visit 14.5 Household respondent reported medical conditions associated with the OPD visit 14.5 Surgical procedures performed during visit 14.5 Types of tests administered during visit 14.5 Types of physician specialist seen 14.5 Facility expense and nine associated sources of payment items 14.5, 18 Doctor expense and nine associated sources of payment items 14.5, 18 VA facility indicator 14.5 1987 EMERGENCY ROOM VISIT AND EXPENDITURE EVENT - LEVEL DATA Date of medical visit 14.5 Number of emergency room visits 13, 14.5, 18 Visit results in inpatient hospital stay 14.5 Household respondent reported medical condition associated with visit 14.5 Type of physician specialist seen during visit 14.5 Surgical procedures performed during visits 14.5 Types of tests administered during visit 14.5 Travel and waiting times 9, 14.5 Mode of transportation to the facility 14.5 VA facility indicator 14.5 Facility expense and nine associated sources of payment items 14.5, 18 Doctor expense and nine associated sources of payment items 14.5, 18 PRIVATE HEALTH INSURANCE CHARACTERISTICS FROM THE HEALTH INSURANCE PLANS SURVEY QUESTIONNAIRES (HELD COVERAGE ONLY) Total health insurance premiums 15* Employer contributions to health insurance premiums 15* Out-of Pocket premium expenses 15* Other contributions toward health insurance premiums 15* Level of health insurance coverage (single, family, etc.) 15* Summary variables of health insurance coverage (including indicators of coverage for basic medical, basic hospitalization, major medical, dental vision and prescription drugs) 15* Indicator of whether employment-related coverage included an HMO or traditional fee-for-service plan 15* Indicator of whether coverage that was not employment-related was an HMO, traditional fee-for-service, or Medigap plan 15* Indicator of group coverage 15* Indicator of self-insurance status of employment-related health-insurance coverage 15* Characteristics of employers and unions providing insurance coverage 15* HIPS: PRIVATE INSURANCE BENEFIT DATABASE Basic and major medical benefits for inpatient hospital, skilled nursing facilities, surgery, physician visits, routine physical exams, well child, psychiatric services, preg-related care, home health and hospice 16* Dental, prescription drugs, vision, or hearing care coverage characteristics and limitations 16* Medicare supplementary coverage (Medigap) including benefits designed to cover Part A and Part B deductibles, Part B coinsurance and outpatient mental health care, restrictions to Medicare reasonable and customary charges, fee schedule allowances and limits on pre-existing conditions 16* HMO characteristics including HMO model type, locations, etc. 16* Cost containment provisions including concurrent and pre admission review, preferred provider organizations, and second surgical opinions 16* Footnote: * Data from NMES Tapes 15 & 16 is not provided on this CD-ROM. These data (Tapes 15 & 16) are from the NMES Health Insurance Plans Survey, which can be linked to the data on this CD-ROM at the insurance policyholder level. INFORMATION ON PREVIOUSLY RELEASED NMES PUBLIC USE TAPES I. Introduction The Agency for Health Care Policy and Research (AHCPR) provides data from the 1987 National Medical Expenditure Survey (NMES-2) for analysis in several different ways. NMES public use tapes represent the principal source of data from NMES-2. These tapes undergo stringent quality control review and are accompanied by full documentation. NMES public use tapes are made available to the public through the National Technical Information Service (NTIS), the Inter-university Consortium for Political and Social Research (ICPSR) and the National Archives Reference Section of the National Archives. Additional information about these NMES tapes is provided in Section II; NTIS ordering information including prices can be found in Section V. In addition to releasing public use tapes, the AHCPR also provides a limited amount of NMES-2 data (not originally planned for public use) on a special request basis. This form of release is constrained to some extent by the availability of funds and programming support. What can be released is also determined by the degree to which confidentiality of persons and institutions might be compromised. Special requests fall into two categories: Research files. Users may request raw files from the NMES-2 data. These data are intended for sophisticated analysts. The files have not been subjected to the same level of editing as the NMES public use tapes. The documentation provided is minimal. Once a research file has been produced for one user, it is available to the public through NTIS and ICPSR. See Section III for details. Merging of NMES-2 data with outside data. Users may request a tape that involves merging of NMES-2 data with other data. The analyst who requests such a tape pays for the data processing costs involved. Such tapes are not distributed to others. information. II. See Section IV for NMES-2 Public Use Tapes NMES-2 public use tapes are available through the National Technical Information Service (NTIS), 5285 Port Royal Road, Springfield, VA 22161, (703) 487-4650. The tapes can also be ordered through the Inter-university Consortium for Political and Social Research (ICPSR) or the National Archives Reference Section. To order through the National Archives, please contact the Reference Section at (202) 501-5579. To order through the Consortium please contact your ICPSR Official Representative or call User Support and Services at (313) 763-5010. NTIS ordering information including prices is provided in Section V. The data for NMES Public Use Tapes 1 through 6 are available on magnetic tape as SAS and EBCDIC files. Beginning with Public Use Tape 8, NMES tapes have been released as EBCDIC files only; however, these tapes also include an EBCDIC file containing the programming statements required to create a SAS data file and a SAS format library. Documentation provided with each NMES tape consists of a description of NMES and the data file, codebooks, technical and programming information, copies of data collection instruments, and information on weights and variance estimation. All files from the NMES Household Survey and Health Insurance Plans Survey (HIPS) can be linked to each other. Similarly, all files from the Institutional Population Component (IPC) and files from the Survey of American Indians and Alaska Natives (SAIAN) can be linked to other files in the same series. AHCPR holds periodic 3-day workshops on the NMES public use tapes. These are generally held in the Washington, DC, metropolitan area. The workshops are free to users, but space is limited. For additional information on NMES public use tapes, including a catalog of data items released on specific tapes, call the Agency for Health Care Policy and Research, Center for General Health Services Intramural Research, at (301) 594-1400/1406. A. NMES Public Use Tapes Released to Date NMES Public Use Tape 1, The 1986 Inventory of Long-Term Care Places (ILTCP). This tape provides a comprehensive listing of nursing and personal care homes and facilities for the mentally retarded in the 50 states and the District of Columbia and constitutes the unedited sampling frame for the Institutional Population Component of NMES. NMES Public Use Tape 2, Institutional Population Component, Phase 1. This tape provides data obtained from a nationally representative sample of nursing homes and facilities for the mentally retarded and their residents on facility and patient characteristics as of 1 January 1987. The facility-level data on this tape have since been replaced by the data on NMES Public Use Tape 6, and the person-level data have been replaced with the data on NMES Public Use Tape 8. NMES Public Use Tape 3, Round 1 Household Survey Population Characteristics and Home Health Provider Data. This tape provides two data sets on a nationally representative sample of the civilian noninstitutionalized population. File 1 contains data on household and individual characteristics of the household sample participants for the first months of 1987, including demographic, insurance, and employment information and indicators of functional status related to the need for and provision of long-term care. File 2 provides data on characteristics and services of home health care providers linked to the household sample population for Round 1 of the Household Survey. File 1 (exclusive of the functional status data) has been replaced with the Round 1 data released on NMES Public Use Tape 13. The functional status data have been replaced with the data released on NMES Public Use Tape 10, and the home health data have been replaced by data on Tape 14.2. NMES Public Use Tape 4, 1987 Prescribed Medicine Event and Person Level Data for Medicare Beneficiaries. This data set from the NMES Household Survey component provides full-year information on prescribed medicines obtained by the civilian noninstitutionalized Medicare population during 1987, as well as related conditions and demographic and insurance information. NMES Public Use Tape 5, Institutional Population Component, Facility Questionnaire Supplement. This tape provides data for a nationally representative sample of nursing and personal care homes and facilities for the mentally retarded on facility characteristics from the Phase 3 facility questionnaire supplement. The data supplement the information elicited in the Phase 1 facility questionnaire (NMES Public Use Tape 6). The data file includes information on current and projected Alzheimer's Disease units in nursing and personal care homes and on accreditation status, resident census, and whether the facilities were part of life-care communities. NMES Public Use Tape 6, Institutional Population Component, Facility Questionnaire Weight Update. This tape updates the facility questionnaire file of the NMES Institutional Population Component, released as Public Use Tape 2, by including a revised weight variable. All other variables remain unchanged. Data items include facility size, ownership, Medicare and Medicaid certification status, services provided, and staffing levels. NMES Public Use Tape 7, Institutional Population Component, Baseline Questionnaire Data for the Current Resident Population, Update. The data on this tape have been merged with NMES Public Use Tape 8. NMES Public Use Tape 8, Institutional Population Component, Baseline Questionnaire Data for the Institutional Population. This tape from the Institutional Population Component (IPC) provides person-level baseline questionnaire data and sampling weights for a nationally representative sample of persons institutionalized at any time in 1987 in nursing and personal care homes and facilities for the mentally retarded. In conjunction with the appropriate weight, the data (including demographic characteristics and health and functional status) can be used to make unbiased national estimates for 1987 for the following institutional populations: (1) all persons who spent one or more nights in an IPC-eligible nursing or personal care home or a facility for the mentally retarded at any time during 1987; (2) persons who were residents of IPC-eligible facilities on January 1, 1987; (3) persons with one or more admissions to a nursing or personal care home during 1987; and (4) all admissions in 1987 to nursing or personal care homes. Because of the relatively small number of annual admissions to facilities for the mentally retarded, the IPC was not designed to provide separate estimates of persons admitted to these facilities during 1987. This data tape replaces personlevel data released on Public Use Tape 2 by providing information for both current residents and persons sampled as admissions. Also included is a revised current resident weight that has been adjusted for sampling frame duplication. NMES Public Use Tape 9, Household Survey, Health Status Questionnaires and Access to Care Supplement Data. This tape contains person-level data from the Household Survey health status questionnaire and the access to care supplement. The data file contains one record each for a total of 30,038 Household Survey respondents who provided information for their entire period of survey eligibility and who responded to a minimum set of items in both the health status questionnaire and the access to care supplement. Children less than age 1 as of December 31, 1987 are excluded from the file. The health status questionnaire elicited information on current and past health status as well as health related behaviors, including care seeking, preventive care, and immunizations for children under age 18. The access to care supplement contained questions about usual sources of medical and dental care, including characteristics of the usual source of care and reasons for the lack of a usual source of care. This tape also contains the link variables necessary to merge characteristics of the usual source of care to home health visit data (Tape 14.2), hospital stays data (Tape 14.4), and ambulatory medical visit data (Tape 14.5). The file also contains basic demographic characteristics for respondents which replace data items previously released in Public Use Tape 3. NMES Public Use Tape 10, Household Survey, Long-Term Care Supplement Data. This tape contains two data files. File 1 contains a person-level record for the 34,459 sampled persons who were eligible for the NMES Household Survey and who responded for their entire period of eligibility. File 1 includes person identifiers, selected demographic data, dates of interview, and weight variables. The demographic data on this file replace corresponding data items previously released in Public Use Tape 3. The population on File 2 is a subset of persons on File 1. File 2 contains long-term care (LTC) supplement data for 33,971 Round 1 respondents and 33,986 Round 4 respondents who were eligible when the supplement was administered. The LTC supplement contained a series of questions relating to functional status, including activities of daily living (ADLs) and instrumental activities of daily living (IADLs), continence, and the use of special equipment, as well as the use of adult day care, senior centers, home-delivered and congregate meals, special transportation, and telephone assurance for those having at least one ADL or IADL difficulty. In addition, the LTC supplement provides information about prior nursing home stays for this group of respondents. These data replace LTC data items previously released on Public Use Tape 3. NMES Public Use Tape 11, Survey of American Indians and Alaska Natives, Round 1 Person-Level Data. This tape was the first public use data file from the NMES SAIAN component. The SAIAN was designed to produce statistically unbiased estimates that are representative of the civilian noninstitutionalized population living on or near Federally recognized reservations and eligible for services provided or supported by the Indian Health Service (IHS). Tape 11 contains one record for each person who was identified and responded in an eligible dwelling unit in Round 1 of the SAIAN, for a total of 7,071 person-level records. Each record includes identifiers, eligibility status indicator, demographic and family characteristics, employment and health insurance status, and sampling weight and variance estimation variables. This file was constructed to permit concatenation of NMES Public Use Tape 3 (which contains demographic, employment, and insurance data for Round 1 of the Household Survey) with the corresponding SAIAN variables. NMES Public Use Tape 13, Household Survey, Population Characteristics and Utilization Data for 1987. This tape from the Household Survey of the 1987 NMES contains person-level characteristics and utilization data collected in Rounds 1-4 of the Household Survey. The tape is intended to serve as the base tape for all NMES Household Survey public use tapes containing full-year 1987 data and contains detailed information on eligibility status and survey administration variables for all persons in the sample. There are two personlevel data files on this tape, both files containing one record for each of 38,446 persons. File 1 contains a combination of edited and constructed variables describing demographic and family relationships, income, disability, employment, health insurance status, and utilization data for all of 1987. File 2 contains the original unedited versions for the edited variables on File 1 as well as imputation flags for the edited and constructed variables on File 1. Full identifier variables and weight and variance estimation variables are included on both files. NMES Public Use Tape 14.1, Household Survey, Prescribed Medicine Data, Calendar Year 1987. This tape from the Household Survey contains information on the use of and expenditures for prescribed medicines for calendar year 1987. The records on this file represent use, expenditures, and sources of payment by persons in the civilian noninstitutionalized population who used prescribed medicines during one or more reference periods during calendar year 1987 and who responded for their entire period of eligibility. The data file contains one record per unique medication per reference period for each eligible person in the Household Survey who reported having purchased or otherwise obtained a prescribed medication during that reference period, for a total of 110,080 records. In addition, each record contains selected personlevel demographic information for the respective user, household respondent reported medical conditions associated with the prescription, frequency of purchase, dates of use, and a range of medication-specific variables such as controlled substance status. The data file also includes full identifier variables, which permit linkages to previously released NMES Household Survey Public Use Tapes and weight and variance estimation variables. This tape can be used to construct aggregate annual person-level variables of expenditures, sources of payment, and other aspects of prescribed medicines for persons with 1987 prescribed medicine use. To make estimates about persons with no prescribed medicine use, this tape must be linked to NMES Public Use Tape 13 or 18. NMES Public Use Tape 14.2, Household Survey, Data for Calendar Year 1987 on Home Health Care and Medical Equipment Purchases and Rentals. This tape from the Household Survey of the 1987 NMES contains information and related documentation on the use of and expenditures and sources of payment for home health care and medical equipment, supplies, and other medical items for calendar year 1987. The data on this tape represent all persons in the civilian noninstitutionalized population who used home health care or medical equipment and supplies for one or more reference periods during calendar year 1987 and who responded for their entire period of eligibility. File 1 (1,682 records) contains one record per unique home health provider for each eligible person in the Household Survey who reported having purchased home health care during the year. In addition to utilization, expenditure, and source-ofpayment information, each record contains selected person-level demographic information, household-reported medical conditions associated with the use of home health care, number of visits by that unique provider, and the beginning and end dates of those provider visits. This file does not include information on informal care giving by friends and family. File 2 (11,010 records) contains one record per type of medical item per round for each eligible person in the Household Survey who reported having purchased, rented, or otherwise obtained such medical item(s) during that reference period. Like the home health file (File 1), this file contains selected person-level demographics and household-reported medical conditions. This tape can be used to construct summary variables of expenditures, sources of payment, and other aspects of utilization of home health care and other medical equipment and supplies. Each data file also includes full identifier variables, which permit linkages to previously released NMES Household Survey Public Use Tapes, and weight and variance estimation variables. NMES Public Use Tape 13 provides information on persons in the Household Survey without home care or without purchase of other medical equipment and supplies in 1987 and annual person-level information on other health services use as well as detailed demographic, employment, and insurance information, round-specific eligibility status indicators, and reference period dates for the entire civilian noninsitutionalized population. NMES Public Use Tape 14.3, Household Survey, Dental Visit Data, Calendar Year 1987. This tape from the Household Survey of the 1987 NMES contains information and related documentation on the use of and expenditures and sources of payment for dental services for calendar year 1987. The records on this file represent dental visits, expenditures and sources of payment for persons in the civilian noninstitutionalized population who used any dental services during calendar year 1987 and who responded for their entire period of eligibility. The data file contains one record per dental visit for a total of 38,429 records. In addition, each record contains selected personlevel demographic information, household respondent reported medical conditions associated with the dental visit, date of visit, and variables describing the type of dental services received. Full identifier variables which permit linkages to previously released NMES Household Survey tapes are also included. This tape can be used to construct summary variables of expenditures, sources of payment, and other aspects of utilization of dental services. NMES Public Use Tape 13 provides information on persons in the Household Survey without dental visits in 1987 as well as detailed demographic, employment, and insurance information for the entire Household Survey sample. NMES Public Use Tape 14.4, Household Survey, Hospital Stays, Calendar Year 1987. This tape contains information on the use of and expenditures and sources of payment for inpatient hospital stays and associated physician expenses for calendar year 1987. The records on this file represent inpatient hospital stays, length of stay, basic facility expenditures and separate physician expenditures, and sources of payment for persons in the civilian noninstitutionalized population who reported having had at least one inpatient hospital stay during calendar year 1987 and who responded for their entire period of eligibility. The data file contains one record per inpatient hospital stay for a total of 5,432 records. In addition, each record contains selected person-level demographic information, household-reported medical conditions and procedures associated with the hospital stay, dates of admission and discharge, the main reason for admission, and the number of nights in the hospital. Full identifier variables that permit linkages to previously released NMES Household Survey tapes are also included. This tape can be used to construct summary variables of expenditures, sources of payment, and other aspects of utilization of inpatient hospital services. NMES Public Use Tape 13 provides information on persons in the Household Survey without inpatient hospital stays in 1987 and annual person-level information on other health services use as well as detailed demographic, employment, and insurance information for the entire Household Survey sample. NMES Public Use Tape 14.5, Household Survey, Ambulatory Medical Visit Data, Calendar Year 1987. This tape from the Household Survey of the NMES contains information and related documentation on the use of and expenditures and sources of payment for ambulatory medical services provided by physicians and other health care providers during calendar year 1987. The data on this tape represent all ambulatory services for persons in the civilian noninstitutionalized population who reported having received at least one ambulatory service during calendar year 1987 and who responded for their entire period of eligibility. The three data files contain data on three types of services. Each record per file represents a unique ambulatory care event: (1) visits and telephone calls to physicians and other providers in settings other than a hospital or home (156,957 records); (2) visits to hospital outpatient departments (20,648 records); and (3) visits to hospital emergency rooms (8,249 records). Excluded from this tape are persons who were admitted and discharged from a hospital on the same day and who had no other ambulatory care visits; these are included on NMES Public Use Tape 14.4 Hospital Stays. In addition to utilization, expenditure, and source-ofpayment information, each record contains selected person-level demographic information, date of visit, household-reported medical conditions and procedures associated with the visit, and the main reason for the visit. This tape can be used to construct summary variables of expenditures, sources of payment, and other aspects of utilization of ambulatory medical services. Full identifier variables that permit linkages to previously released NMES Public Use Tapes are also included on each data file. NMES Public Use Tape 13 provides information on persons in the Household Survey without ambulatory medical services in 1987 and annual person-level information on other health services use as well as detailed demographic, employment, and insurance information for the entire Household Survey sample. NMES Public Use Tape 15, Policyholders of Private Health Insurance: Premiums, Payment Sources, and Type and Source of Coverage. This tape is the first release from the Health Insurance Plans Survey (HIPS) of the 1987 NMES. HIPS is a follow-up to the NMES Household Survey and was designed to verify health insurance status provided by the NMES Household Survey respondents as well as to provide supplementary information on private health insurance coverage. The tape provides information and related documentation on private health insurance in force at the end of calendar year 1987 for employmentrelated and other coverage of all policyholders in the U.S. noninstitutionalized civilian population. The tape contains two data files, one for employment-related insurance (File 1) and one for insurance that is purchased directly from insurance carriers or associations (File 2). With this tape it is possible to make estimates of total premiums for private health insurance as well as estimates of employer and out-of-pocket contributions for these premiums. Records on each data file contain selected person-level demographic information for the respective policyholder as well as some characteristics of the policyholder's health insurance coverage and the provider of employmentrelated insurance (File 1). The persons represented on these data files are not the universe of persons with private health insurance but rather policyholders of private health insurance. The link variables provided in both Files 1 and 2 enable linkage of policyholders to their person and socioeconomic characteristics provided on NMES Household Survey public use tapes (e.g., Public Use Tape 13). The present public use tape does not provide linkage to the dependents of policyholders of private health insurance coverage. In particular, it is not possible to use information on dependents from the coverage data available in Public Use Tape 13 to link dependents such as family members to policyholders with records on the current tape (these links will be provided on NMES Tape 24). In addition, Tape 15 does not contain benefit information abstracted from policy booklets collected as part of the HIPS (this information is provided for policyholders on NMES Tape 16). The premium variables provided on Tape 15 will be updated with the release of NMES Tape 15U. NMES Public Use Tape 16, Health Insurance Plans Survey Data: Private Insurance Benefit Database and Linkages to Household Survey Policyholders. This tape from the Health Insurance Plans Survey (HIPS) of the 1987 NMES contains the Private Health Insurance Benefit Database and related documentation that describe the private insurance benefits of persons in the NMES Household Survey and the Survey of American Indians and Alaska Natives (SAIAN). The 17 data files on this tape include two link files (Files 1 and 2) and the Benefit Database (Files 3-17), which includes unedited data derived from the abstracted contents of all descriptions of private health insurance policies obtained in the HIPS. The link files link the abstracted benefit data to Household Survey policyholders and can be used to make estimates of the types of coverage in force at the end of 1987. The link files contain all policyholders in the Household Survey who were eligible and responded during the Round 4 interview and who also responded for their entire period of eligibility during 1987. The records in the two link files correspond exactly to the records in the two files on Public Use Tape 15, which contains data on premiums and other characteristics of coverage of Household Survey policyholders (File 1 links to holders of employment-related insurance, and File 2 links to policyholders of insurance purchased directly from an insurer or association). Files 1 and 2 on the present tape also permit linkage to the personal and socioeconomic characteristics on other NMES Household Survey public use tapes (e.g., Tape 13) for these policyholders. The Benefit Database includes a header file (File 3), 10 section files (Files 4-13), and 4 schedule files (Files 14-17). The section files include information on the types of coverage abstracted from policy descriptions (HMO, basic coverage, cost-containment basic coverage, major medical, cost-containment major medical, dental care, prescription drugs, vision care, hearing care, and Medigap/retiree coverage). The schedule files include specific deductible, coinsurance, and maximum benefit provisions. Future public use tapes planned from the HIPS will permit linkage of the Benefit Database (Files 3-17) to dependents of policyholders in the Household Survey (NMES Tape 24) and to persons in the SAIAN. The future link files will also permit analysis of health insurance options offered by employers and unions. Documentation includes the Insurance Abstraction Manual and Users' Guide, which describes coding procedures and conventions used for the coding of health insurance policies. NMES Public Use Tape 17, Institutional Population Component, Facility Use and Expenditure Data for Nursing and Personal Care Home Residents. This tape from the Institutional Population Component (IPC) of the 1987 NMES contains personlevel, institutional stay-level, and bill-level data for a nationally representative sample of persons institutionalized in nursing and personal care homes. The IPC includes a sample of persons residing in or admitted to nursing and personal care homes and facilities for the mentally retarded at any time in calendar year 1987. The three data files on this tape contain data for sample persons (SPs) who were sampled either as current residents (residents on January 1, 1987) or as new admissions (admitted between January 1 and December 31, 1987) in nursing and personal care homes only. File 1 (a person-level file) contains variables pertaining to survey administration, demographic characteristics (age, race, and sex), date of admission, vital status, residence history before admission to the sampled facility, education, veteran status, prior lifetime-use of long-term care, family characteristics, insurance coverage, home ownership, income, and medical conditions. File 2 (a stay-level file) contains information on the SP's 1987 institutional stay(s), including episodes of institutional care, dates of the stays, and characteristics of the institution of residence such as facility size, ownership, certification status, and location. Stays in other types of facilities (e.g., acute care hospitals) and in the community are not included (but will be on a future tape (Tape 27). File 3 (a bill-level file) contains expenditures and sources of payment for both basic and ancillary services provided in nursing and personal care home facilities. NMES Public Use Tape 18, Household Survey, Expenditures, Sources of Payment, and Population Data for 1987. This tape is one in a series of public use tapes from the Household Survey (HS) of the 1987 National Medical Expenditure Survey (NMES). This tape contains 38,446 person-level records with information on health care utilization, expenditures, and sources of payment; 26 sources of income; assets; tax filing; and demographic characteristics including month-specific insurance variables and HMO indicators. The tape also contains detailed information on eligibility status, record and person identifiers, link variables, weights and variance estimation, and other survey administration variables for all persons in the sample. This tape supplements Public Use Tape 13 (Household Survey - Population Characteristics and Utilization Data for 1987) and provides data not previously released. Most of the person characteristics variables from Tape 13 are not duplicated here; for the convenience of the user, the annual utilization variables are duplicated on this tape, along with person-level expenditure and sources-of-payment information released for the first time. Event-level expenditure and sourceof-payment information was released on Public Use Tapes 14.1-14.5, which can be linked to this file. In addition, Tape 18 can be used to make familylevel estimates. NMES Public Use Tape 20P, Survey of American Indians and Alaska Natives Preliminary Population Characteristics for 1987. This public use tape contains person-level data from the NMES SAIAN component. The SAIAN was designed to yield national estimates for the civilian noninstitutionalized U.S. population living on or near Federally recognized reservations and eligible for services provided or supported by the Indian Health Service (IHS). The tape contains detailed information on eligibility status, interview dates, demographic characteristics, employment and insurance variables, link variables, and other survey administration variables for all persons (7,533) in the sample in Rounds 1-3 of the SAIAN. This file was constructed to permit concatenation of NMES Public Use Tape 13 with the corresponding SAIAN variables. The Round 1 person characteristics previously released on Public Use Tape 11 are being replaced by the data contained on this tape. This tape represents a preliminary release of these data; as such, it has not been subjected to the standard quality control procedures applied to NMES public use tapes. The final version, Public Use Tape 20, will serve as the base data for all NMES SAIAN public use tapes containing full-year 1987 data. Tape 20 will also contain data on final annual measures of medical utilization and disability days. The data on Tape 20P do not include information on medical utilization, expenditures, and sources of payment associated with each of the health care services or goods used during the survey year by each sampled person. This information, together with details associated with each health care event, will be available in Public Use Tapes 23.1-23.5. NMES Public Use Tape 21P, Survey of American Indians and Alaska Natives, Preliminary Health Status and Access to Care Data, Calendar Year 1987. This tape contains person-level data from the NMES SAIAN component's health status questionnaires and the access to care supplement. The SAIAN was designed to yield national estimates for care the civilian noninstitutionalized U.S. population living on or near Federally recognized reservations and eligible for services provided or supported by the Indian Health Service (IHS). This tape contains one record each for a total of 5,695 SAIAN respondents who provided information for their entire period of survey eligibility and responded to a minimum set of items in both the health status questionnaires and the access to care supplement. Children less than age 1 as of December 31, 1987, are excluded from this file. The health status questionnaire elicited information on current and past health status, including acute and chronic conditions, vision and hearing, dental status, mental health and functional ability, as well as health-related behaviors, including care seeking and preventive care, and immunizations for children under age 18. The access to care supplement contained questions about usual sources of medical and dental care, including characteristics of the usual source of care and reasons for the lack of a usual source of care. This file was constructed to permit concatenation of NMES Public Use Tape 9 with the corresponding SAIAN variables. The file also contains basic demographic characteristics for respondents (age, race and sex) as established in the SAIAN, which replace data items previously released in Public Use Tape 11 (Round 1 SAIAN data). This tape represents a preliminary release of these data; as such, it has not been subjected to the standard quality control procedures applied to NMES public use tapes. These data will be released in final form on NMES Public Use Tape 21. Tape 21 will also contain the link variables necessary to merge characteristics of the usual source of care to the home health visit data (Tape 23.2), hospital stays data (Tape 23.4), and ambulatory medical visit data (Tape 23.5). NMES Public Use Tape 23.1P, Survey of American Indians and Alaska Natives, Preliminary Prescribed Medicine Data, Calendar Year 1987. This tape from the NMES SAIAN component contains information and related documentation on the use of prescribed medicines during 1987 by the civilian noninstitutionalized population living on or near Federally recognized reservations or in Alaska and eligible for services provided or supported by the Indian Health Service (IHS). The data file contains one record per unique medication per reference period for each of these persons who reported having purchased or otherwise obtained a prescribed medication during that reference period and who responded for their entire period of eligibility (10,191 records). In addition, each record contains selected person-level demographic information for the respective user, medical conditions associated with the prescription, frequency of purchase, dates of use, and the NCHS drug code for the medication. When taken together, the set of records included for each person in this file represent all instances in which the person purchased or otherwise obtained a medication (including refills) during 1987. The file can be used to construct summary variables of utilization of prescribed medicines. Only SAIAN-eligible persons with prescribed medicine use who responded for their entire period of eligibility are included on this tape. This file was constructed to permit concatenation of NMES Public Use Tape 14.1 with the corresponding SAIAN variables. This tape represents a preliminary release of data; as such, it has not been subjected to the standard quality control procedures applied to NMES public use tapes. These data will be released in final form on NMES Public Use Tape 23.1. Tape 23.1 will also contain additional descriptive variables (including the generic equivalent of drugs), whether the drugs were obtained at an IHS or non-IHS sponsored provider, and edited expenditure and source- of-payment variables. NMES Tape 20 will provide information on persons in SAIAN without use of prescribed medicines in 1987 as well as detailed person-level information on demographics, employment, and insurance coverage (these data were released in preliminary form in Tape 20P). NMES Public Use Tape 23.2P, Survey of American Indians and Alaska Natives, Preliminary Data for Calendar Year 1987 on Home Health Care, Medical Equipment Purchases and Rentals, and Traditional Medicine. This tape from the NMES SAIAN component contains information and related documentation on the use of formal home health care; the purchase and rental of medical equipment, supplies and other medical items; and traditional medicine during 1987 by the civilian noninstitutionalized population living on or near Federally recognized reservations or in Alaska and eligible for services provided or supported by the Indian Health Service (IHS). The tape provides three data files. The data file on home health services (File 1) contains one record per unique formal home care provider per reference period for each eligible person in the SAIAN who reported receiving home health services (296 records). File 2 contains one record per type of medical item per reference period for each eligible person in the SAIAN who reported having purchased, rented, or otherwise obtained such items during that round (1,315 records). File 3 contains one record for each use (one or more times) of a unique practitioner of traditional medicine services per reference period for each eligible person in the SAIAN who reported receiving at least one traditional medicine service in at least one round (340 records). All files on the tape also contain household-reported medical condition and selected person-level demographic variables. Only SAIAN-eligible persons with home health use, purchase or rental of medical equipment, and traditional medicine use who responded for their entire period of eligibility are included on this tape. This tape represents a preliminary release of data; as such, it has not been subjected to the standard quality control procedures applied to NMES public use tapes. These data will be released in final form on NMES Public Use Tape 23.2. Tape 23.2 will also contain additional descriptive variables on these services, edited expenditure and source of payment variables, an indicator of whether the service was provided by an IHS or non-IHS sponsored provider (on Files 1 and 2 only), and an edited variable for the number of visits (on File 1 only). NMES Tape 20 will provide information on persons in SAIAN without use of home health services, other medical equipment, or traditional medicine in 1987 (this data was released in preliminary form in Tape 20P). NMES Public Use Tape 23.3P, Survey of American Indians and Alaska Natives, Preliminary Dental Visit Data, Calendar Year 1987. This tape from the NMES SAIAN component contains information and related documentation on dental visits during 1987 by the civilian noninstitutionalized population living on or near Federally recognized reservations or in Alaska and eligible for services provided or supported by the Indian Health Service (IHS). The data file contains one record per dental visit for each eligible person in the SAIAN who reported having a dental visit during the year and who also responded for their entire period of eligibility during 1987 (4,417 records). In addition, each record contains basic person-level demographic information for the sample person, type of service obtained during the dental visit, dates of service, and medical conditions as reported by the household respondent if the visit was due to accident or injury. All dental visits reported by respondents are included, regardless of whether the visit was to an IHS or non-IHS sponsored provider. This file was constructed to permit concatenation of NMES Public Use Tape 14.3 with the corresponding SAIAN variables. This tape represents a preliminary release of data; as such, it has not been subjected to the standard quality control procedures applied to NMES public use tapes. These data will be released in final form on NMES Public Use Tape 23.3. Tape 23.3 will also contain additional descriptive dental visit variables including information on type of dental provider, edited expenditure and source of payment variables, and an indicator of whether the dental visit was to an IHS or non-IHS sponsored provider. NMES Tape 20 will provide information on persons in SAIAN without use of dental services in 1987 as well as detailed person-level information on demographics, employment, and insurance coverage (these data were released in preliminary form in Tape 20P). NMES Public Use Tape 23.4P, Survey of American Indians and Alaska Natives, Preliminary: Hospital Stays, Calendar Year 1987. This tape from NMES SAIAN component contains information and related documentation on the use of inpatient hospital stays during 1987 by the civilian noninstitutionalized population living on or near Federally recognized reservations or in Alaska and eligible for services provided or supported by the Indian Health Service (IHS). The data file contains one record for each hospital stay for each eligible person in the SAIAN who reported having had at least one hospital stay during 1987 and who also responded for their entire period of eligibility during 1987 (1,134 records). Each record contains selected person-level demographic information for the respective patient, household-reported medical conditions and procedures associated with the hospitalization, and information on length of stay. This file was constructed to permit concatenation of NMES Public Use Tape 14.4 with the corresponding SAIAN variables. This tape represents a preliminary release of data; as such, it has not been subjected to the standard quality control procedures applied to NMES public use tapes. These data will be released in final form on NMES Public Use Tape 23.4. In addition to edited versions of some of the unedited variables on the current tape, Tape 23.4 will also contain additional descriptive variables and edited expenditure and source-of-payment variables. NMES Tape 20 will provide information on persons in SAIAN without hospital stays in 1987 as well as detailed person-level information on demographics, employment, and insurance coverage (these data were released in preliminary form in Tape 20P). NMES Public Use Tape 23.5P, Survey of American Indians and Alaska Natives, Preliminary Ambulatory Medical Visit Data, Calendar Year 1987. This tape from the NMES SAIAN component contains information and related documentation on the use of ambulatory medical services during 1987 by the civilian noninstitutionalized population living on or near Federally recognized reservations or in Alaska and eligible for services provided or supported by the Indian Health Service (IHS). This tape contains three data files: (1) visits and telephone calls to physicians and other providers in settings other than a hospital or at home (8,031 records); (2) visits to hospital outpatient departments, regardless of provider type (10,690 records); and (3) hospital emergency room visits, regardless of provider type (1,917 records). Only SAIAN-eligible persons with ambulatory use who responded for their entire period of eligibility are included on this tape. All ambulatory medical services reported by respondents are included, regardless of whether they were provided at an IHS or non-IHS sponsored facility. A record on any of these data files represents a unique ambulatory visit. This file was constructed to permit concatenation of NMES Public Use Tape 14.5 with the corresponding SAIAN variables. In addition, each record contains selected person-level demographic information for the respective user, dates of visits, household respondent-reported medical conditions associated with the visit, and variables such as types of procedures performed and the main reason for the visit. This tape represents a preliminary release of data; as such, it has not been subjected to the standard quality control procedures applied to NMES public use tapes. These data will be released in final form on NMES Public Use Tape 23.5. Tape 23.5 will also contain additional descriptive variables, edited expenditure and source-of-payment variables, and an indicator of whether the services were provided at an IHS or non-IHS sponsored facility. NMES Tape 20 will provide information on persons in SAIAN without use of ambulatory medical services in 1987 as well as detailed person-level information on demographics, employment, and insurance coverage (these data were released in preliminary form in Tape 20P). III. NMES-2 Research Files AHCPR releases to the research community a limited amount of NMES-2 data on an individual request basis. These data are not otherwise be released as NMES public use tapes. The requests for data are subject to resource constraints. Research files are intended for sophisticated users who are familiar with the NMES public use tapes and have experience analyzing complex survey data. These files have not been subjected to the same level of editing and imputation as the NMES public use tapes and generally include raw data. On occasion no sampling weights accompany the files. These data should be analyzed and interpreted with care. The documentation provided is extremely limited. Research files are provided in this form because of the limited audience of users for these data. NMES-2 research files are released to individual users as EBCDIC files on magnetic tape. Once a research file has been produced for one user, it is available to the public through NTIS and ICPSR at normal distribution costs. IV. Merging Secondary Data and the NMES-2 Public Use Files AHCPR will also merge secondary data items to NMES public use tapes and research files, and make some of these data available to researchers. These files must be developed to comply with the statutory requirements to keep the identity of respondents, individuals and institutions, that might be identified, confidential. Examples of secondary data items that might be merged to public NMES data are: selected items from the Area Resources File, State-level characteristics, and indicators of urban/rural status. AHCPR also provides cross-tabulations and executes other fully functioning customized software using non-public-use data. Examples of the latter are (1) a program that uses person characteristics (income, family size, etc.) from the Household Survey and State identifiers to create a variable with state tax rates, and (2) SAS programming code to execute a model that includes variables never to be released (e.g., Census region for the Institutional Population Component). The individual user who makes the request for merging secondary data with NMES-2 public use tapes pays the data processing costs involved in: merging the data, investigating issues of confidentiality, and executing the software. Only the person requesting the file will receive the merged and encrypted file (or computer output). The files will be provided as either EBCDIC or SAS files. For details see the Guide to NMES-2 Special Data Requests, a document describing the procedures, costs, and limitations involved. The document is available from the AHCPR, Center for General Health Services Intramural Research, (301) 594-1400/1406. V. Ordering Information A. NTIS Ordering Information NMES public use tapes and research files are released to the public through the National Technical Information Service (NTIS), 5285 Port Royal Road, Springfield, VA 22161, (703) 487-4650. To expedite processing by the NTIS, please complete the attached form (last page in this document) and include it with your NTIS order request. The data for NMES Public Use Tapes 1 through 6 are available on magnetic tape as SAS and EBCDIC files. Beginning with Public Use Tape 8, NMES tapes are released as EBCDIC files only; however, these tapes also include an EBCDIC file containing the programming statements required to create a SAS data file and a SAS format library. Related documentation provided with each NMES tape consists of a description of NMES and the data file, codebooks, technical and programming information, copies of data collection instruments and information on weights and variance estimation. Prompt access to the documentation only can also be obtained by calling the Agency for Health Care Policy and Research, Center for General Health Services Intramural Research at (301) 594-1406. There is no charge for this service. 1. NMES Public Use Tapes NMES Public Use Tape 1, The 1986 Inventory of Long Term Care Places (ILTCP) NTIS accession number PB88-122387: price: $15.95. SAS file documentation only; NTIS accession number PB88-122361: price: $15.95. EBCDIC file documentation only; NTIS accession number PB88-122379: documentation; price: $210. SAS data files and NTIS accession number PB88-122353: documentation; price: $210. EBCDIC data files and NMES Public Use Tape 2, Institutional Population Survey, Phase 1 NTIS accession number PB89-178495: SAS file documentation only; price: $49.95-hard copy, $6.95-microfiche. NTIS accession number PB89-178479: EBCDIC file documentation only; price: $49.95-hard copy, $6.95-microfiche. NTIS accession number PB89-178487: documentation; price: $325. SAS data files and NTIS accession number PB89-178461: documentation; EBCDIC data files and price: $325. NMES Public Use Tape 3, Round 1 Household Survey Population Characteristics and Home Health Provider Data NTIS accession number PB90-112988: SAS file documentation only; price: $67-hard copy, $16.50-microfiche. NTIS accession number PB90-112970: EBCDIC file documentation only; price: $61.95-hard copy, $15.50-microfiche. NTIS accession number PB90-500232: documentation; price: $340. SAS data files and NTIS accession number PB90-500224: documentation; EBCDIC data files and price: $340. NMES Public Use Tape 4, 1987 Prescribed Medicine Event and Person Level Data for Medicare Beneficiaries NTIS accession number PB90-101254: SAS file documentation only; price: $45-hard copy, $15-microfiche. NTIS accession number PB90-101247: EBCDIC file documentation only; price: $45-hard copy, $15-microfiche. NTIS accession number PB90-501362: documentation; price: $340. SAS data files and NTIS accession number PB90-501388: documentation; EBCDIC data files and price: $340. NMES Public Use Tape 5, Institutional Population Component, Facility Questionnaire Supplement NTIS accession number PB91-110480: SAS file documentation only; price: $39-hard copy, $11-microfiche. NTIS accession number PB91-110494: EBCDIC file documentation only; price: $39-hard copy, $11-microfiche. NTIS accession number PB91-505453: documentation; price: $340. SAS data files and NTIS accession number PB91-505461: documentation; EBCDIC data files and price: $340. NMES Public Use Tape 6, Institutional Population Component, Facility Questionnaire Weight Update NTIS accession number PB91-146266: SAS file documentation only; price: $39-hard copy, $11-microfiche. NTIS accession number PB91-146258: EBCDIC file documentation only; price: $39-hard copy, $11-microfiche. NTIS accession number PB91-506683: documentation; price: $340 SAS data files and NTIS accession number PB91-506675: documentation; EBCDIC data files and price: $340 NMES Public Use Tape 8, Institutional Population Component, Baseline Questionnaire Data for the Institutional Population NTIS accession number PB91-146274: documentation only; price: $45-hard copy, $15-microfiche. NTIS accession number PB91-506691: price: $340. tape and documentation; NMES Public Use Tape 9, Household Survey, Health Status Questionnaires and Access to Care Supplement Data NTIS accession number PB91-190165: documentation only; price: $53-hard copy, $17-microfiche. NTIS accession number PB91-507533: price: $340. tape and documentation; NMES Public Use Tape 10, Household Survey, Long-Term Care Supplement Data NTIS accession number PB91-221739: documentation only; price: $60-hard copy, $17-microfiche. NTIS accession number PB91-509828: price: $340. tape and documentation; NMES Public Use Tape 11, Survey of American Indians and Alaska Natives, Round 1 Person-Level Data NTIS accession number PB91-227926: documentation only; price: $60-hard copy, $17-microfiche. NTIS accession number PB91-509885: price: $340. tape and documentation; NMES Public Use Tape 13, Household Survey, Population Characteristics and Utilization Data for 1987 NTIS accession number PB92-100189: documentation only; price: $73-hard copy, $27-microfiche. NTIS accession number PB92-500057: price: $360. tape and documentation; NMES Public Use Tape 14.1, Household Survey, Prescribed Medicine Data, Calendar Year 1987 NTIS accession number PB92-139096: documentation only; price: $59-hard copy, $26-microfiche. NTIS accession number PB92-501287: price: $480. tape and documentation; NMES Public Use Tape 14.2, Household Survey, Data for Calendar Year 1987 on Home Health Care and Medical Equipment Purchases and Rentals NTIS accession number PB93-100279: documentation only; price: $84-hard copy, $27-microfiche. NTIS accession number PB93-500213: price: $480. tape and documentation; NMES Public Use Tape 14.3, Household Survey, Dental Visit Data, Calendar Year 1987 NTIS accession number PB92-155423: documentation only; price: $59-hard copy, $19-microfiche. NTIS accession number PB92-501865: price: $480. tape and documentation; NMES Public Use Tape 14.4, Household Survey, Hospital Stays, Calendar Year, 1987 NTIS accession number PB92-169671: documentation only; price: $73-hard copy, $26-microfiche. NTIS accession number PB92-503150: price: $360. tape and documentation; NMES Public Use Tape 14.5, Household Survey, Ambulatory Medical Visit Data, Calendar Year 1987 NTIS accession number PB92-218510: documentation only; price: $91-hard copy, $32-microfiche. NTIS accession number PB92-504307: price: $480. tape and documentation; NMES Public Use Tape 15, Policyholders of Private Health Insurance: Premiums, Payment Sources, and Type and Source of Coverage NTIS accession number PB92-221852: documentation only; price: $77-hard copy, $27-microfiche. NTIS accession number PB92-504349: price: $480. tape and documentation; NMES Public Use Tape 16, Health Insurance Plans Survey Data: Private Insurance Benefit Database and Linkages to Household Survey Policyholders NTIS accession number PB93-213940: documentation only; price: $147-hard copy, $72-microfiche. NTIS accession number PB93-506400: price: $360. tape and documentation; NMES Public Use Tape 17, Institutional Population Component, Facility Use and Expenditure Data for Nursing and Personal Care Home Residents NTIS accession number PB93-192763: documentation only; price: $91-hard copy, $32-microfiche. NTIS accession number PB93-506046: price: $360. tape and documentation; NMES Public Use Tape 18, Household Survey, Expenditures, Sources of Payment, and Population Data for 1987. NTIS accession number PB94-104619: documentation only; price: $xx -hard copy, $ -microfiche. NTIS accession number PB94-500071: price: $360. tape and documentation; NMES Public Use Tape 20P, Survey of American Indians and Alaska Natives, Preliminary Population Characteristics for 1987 NTIS accession number PB94-xxxxx: price: xx. documentation only; NTIS accession number PB94-500139 tape and documentation; price: $360. NMES Public Use Tape 21P, Survey of American Indians and Alaska Natives, Preliminary Health Status Questionnaires and Access to Care Supplement (Preliminary) Data, Calendar Year 1987 NTIS accession number PB93-213858: price: xx. documentation only; NTIS accession number PB93-506228: price: $360. tape and documentation; NMES Public Use Tape 23.1P, Survey of American Indians and Alaskan Natives, Preliminary Prescribed Medicine Data, Calendar Year 1987 NTIS accession number PB93-213981: documentation only; price: $77-hard copy, $27-microfiche NTIS accession number PB93-506871: price: $360. tape and documentation; NMES Public Use Tape 23.2P, Survey of American Indians and Alaskan Natives, Preliminary Data for Calendar Year 1987 on Home Health Care, Medical Equipment Purchases and Rentals, and Traditional Medicine NTIS accession number PB93-233708: documentation only; price: $77-hard copy, xx-microfiche. NTIS accession number PB93-507036: price: $360. tape and documentation; NMES Public Use Tape 23.3P, Survey of American Indians and Alaska Natives, Preliminary Dental Visit Data, Calendar Year 1987 NTIS accession number PB93-214005: documentation only; price: $61-hard copy, $19.50-microfiche NTIS accession number PB93-506897: price: $360. tape and documentation; NMES Public Use Tape 23.4P, Survey of American Indians and Alaska Natives, Preliminary: Hospital Stays, Calendar Year 1987 NTIS accession number PB93-218428: documentation only; price: $84-hard copy, $27-microfiche. NTIS accession number PB93-505962: price: $360. tape and documentation; NMES Public Use Tape 23.5P, Survey of American Indians and Alaskan Natives, Preliminary Ambulatory Medical Visit Data, Calendar Year 1987 NTIS accession number PB93-218451: documentation only; price: $77-hard copy, $27-microfiche. NTIS accession number PB93-506970: price: $360. tape and documentation; 2. NMES Research Files As of mid-October 1993, NTIS has not released any NMES research files. VARIANCE ESTIMATION PROGRAMS FOR USE WITH COMPLEX SURVEY DATA I. Variance Estimation Procedures All component surveys of the National Medical Expenditure Survey (NMES) are characterized by complex sample designs such as stratification, clustering, and disproportionate sampling. This departure from simple random sampling assumptions requires special consideration with regard to variance estimation and analysis. Several methods for approximating sampling variances, which incorporate the components of a complex survey design, have been developed and can be considered for application to NMES data. The three most generally accepted and frequently used techniques are the method of balanced repeated replication (BRR), the "jackknife" method, and the Taylor series linearization method. These variance estimation strategies have been incorporated as procedures in several of the widely used statistical packages. II. Variance Estimation Procedures and NMES Each NMES public use tape is released with the variables necessary to estimate variances using the Taylor series linearization method. See the tapespecific NMES documentation for variable names. Sections III-V lists several variance estimation programs that are appropriate for use with complex survey data: III. Mainframe A. The following programs were developed by the Research Triangle Institute (Research Triangle Park, N.C.) and all use the Taylor Series linearization method for complex survey data. SESUDAAN: Standard Errors Program for Computing of Standardized Rates from Sample Survey Data, accessible through SAS, and developed by the Research Triangle Institute (Shah, 1981a). Uses Taylor series linearization method for complex survey data. RATIOEST: Standard Errors Program for Computing Ratio Estimates from Sample Survey Data, accessible through SAS, and developed by the Research Triangle Institute (Shah, 1981b). Uses Taylor series linearization method for complex survey data. SURREGR: Standard Errors of Regression Coefficients from Sample Survey Data accessible through SAS, and developed by the Research Triangle Institute (Holt, 1982). Uses Taylor series linearization method for complex survey data. RTILOGIT: Survey Data Analysis Software for Logistic Regression, used in conjunction with supplementary SAS procedure, PROC LOGIST, and developed by the Research Triangle Institute (Shah et al., 1984). Uses the Taylor series linearization method SUDAAN: SUrvey DAta ANalysis software. Incorporates the above four packages plus several new ones. Can read in both SAS and text data files, and was developed by Research Triangle Institute (Shah, Barnwell, Hunt, & La Vange, 1991). B. The following programs were developed by Westat, Inc., and use either the balanced half-sample replication (BRR) or Jackknife replication methods for complex survey data. WESVAR: Computes basic survey estimates and associated sampling errors, as well as confidence intervals and Chi-square tests of independence, and is accessible through SAS (WESTAT, INC., 1989). Uses either balanced repeated half-sample replication (BRR) or jacknife replication methods for complex survey data. WESREG: Computes sampling errors of multiple regression estimates, and is accessible through SAS developed by WESTAT, INC. (1989). Use the balanced half sample replication technique. WESLOG: Computes sampling errors for the logistic model, and is accessible through SAS developed by WESTAT, INC. (1989). Uses the balanced repeated replication method. C. The following were developed by the Survey Research Center of the Institute for Social Research, University of Michigan. PSALMS: Sampling Error Analysis procedure, accessible through OSIRIS IV (Statistical Analysis and Data Management Software Systems Package), and developed by the Survey Research Center of the Institute for Social Research, University of Michigan (Van Eck, 1979). Uses the Taylor series linearization method. REPERR: Repeated Replication Sampling Error analysis procedure, accessible through OSIRIS IV, and developed by the Survey Research Center of the Institute for Social Research, University of Michigan (Van Eck, 1979). Uses any of three repeated replication procedures: balanced half-sample, jackknife, or user specified. D. Other mainframe variance estimation programs include the following: SUPER CARP: Cluster Analysis and Regression Program developed by the Survey Section of the Statistical Laboratory at Iowa State University (Hidirouglou, Fuller, Hickman, 1980). Uses Taylor series linearization method. Reads in text data files only. HESBRR: Health Examination Survey Variance and Crosstabulation Program, developed by the National Center for Health Statistics (Jones, 1983). Uses balanced half-sample method. CPLX/VPLX: Developed by the Census Bureau. IV. For the PC: SUDAAN: PC and VAX version of the mainframe SUDAAN package described above and developed by Research Triangle Institute (Shah, Barnwell, Hunt, and La Vange, 1991). PC CARP: PC version of the mainframe SUPER CARP package described above, but with added features, and has a supplementary logistic regression module, and developed by Survey Section of the Statistical Laboratory at Iowa State University (Fuller, et al, 1989). CPLX/VPLX: PC and VAX version of mainframe CPLX/VPLX package mentioned above and developed by the Census Bureau.. V. Estimation capabilities of several variance estimation programs Computes standard errors for: Means Logistic proportions regression Program coefficients Ratios, rates totals Regression coefficients SESUDAAN X SUDAAN X X X X SUPER CARP X X X PC CARP X X X WESVAR X X HESBRR X X PSALMS X X RATIOEST X X SURREGR X REPERR X WESREG X RTILOGIT X WESLOG X VI. References Fuller, W.A., W. Kennedy, D. Schell, G. Sullivan and H.J. Park. (1989). PC CARP (Version 1.3). Statistical Laboratory, IOWA State University , Ames, Iowa Hidiroglou, M.A., Fuller, W.A. and Hickman R. (1980). SUPERCARP (6th ed.) Ames, Iowa, Iowa State University, Survey Section, Statistical Laboratory. Holt, M.M., (1982). SURREGR: Standard errors of regression coefficients from sample survey data. Technical report, Research Triangle Park, North Carolina: Research Triangle Institute. Jones, G.K., (1983). HESBRR (HES Variance and Crosstabulation Program, Version 3). Unpublished report, Hyattsville, Maryland: National Center for Health Statistics. Shah, B.V., (1981a). SESUDAAN: Standard Errors Program for Computing of Standardized Rates From Sampled Data. Technical report, Research Triangle Park, North Carolina: Research Triangle Institute. Shah, B.V., (1981b). RATIOEST: Standard errors program for computing ratio estimates for sample survey data. Technical report, Research Triangle Park, North Carolina: Research Triangle Institute. Shah, B.V., B.G. Burnwell, P.N. Hunt and L.M. La Vange, (1992). SUDAAN User's Manual. Professional Software for SUrvey DAta ANalysis for Multi-stage Sample Design, Release 6.0, Research Triangle Park, N.C. Shah, B.V., R.E. Folsom, F.E. Harrell, C.N. Dillardz. (1984). RTILOGIT: Survey Data Analysis Software for Logistic Regression. Technical report, Research Triangle Park, North Carolina: Research Triangle Institute. Van Eck, N., (1979). OSIRIS IV Users Manual: 15th edition. Survey Research Center, Institute for Social Research, The University of Michigan, Ann Arbor, MI. WESTAT, Inc. 1989. The WESREG Procedure, Rockville, Maryland. WESTAT, Inc. 1989. The WESLOG Procedure, Rockville, Maryland. WESTAT, Inc. 1989. The WESVAR Procedure, Rockville, Maryland. NATIONAL HEALTH INTERVIEW SURVEY PROCEDURE CODES FOR USE WITH THE NMES HOUSEHOLD DATA INDEX OF OPERATIONS AND NON-OPERATIVE PROCEDURES, FOR HIS CLASSIFICATION OF OPERATIONS AND NON-OPERATIVE PROCEDURES, FOR HIS I. INDEX OF OPERATIONS AND NON-OPERATIVE PROCEDURES, FOR HIS Note: See Part II of this section for the Classification of Operations and Non-Operative Procedures used by HIS, with a general explanation of its content and form. In the following Index of Operations and Non-Operative Procedures, for HIS, names of operations and procedures are listed in alphabetical order. Operations for certain specified conditions are listed in the alphabetical order of the condition. For example: Cataract operation 13; Harelip operation 27; Pilonidal cyst operation 86. Codes for operations which are classified according to site--and which are not classified elsewhere to a particular kind of operation or condition--are found by site under the heading "OPERATION, NEC." The abbreviation "OP NEC," seen frequently in this Index, means "operation not elsewhere classified." Operations are classified in nine groups of procedures: 1-Incision 2-Excision 3-Amputation 4-Introduction 5-Endoscopy 6-Repair 7-Destruction 8-Suturing 9-Manipulation These procedures are defined as follows: 1. Incision is a cutting into; an opening. used are: - otomy--a cutting out (to cut into) ostomy--a cutting into to form an opening centesis--a puncture (aspiration) 2. Excision is a cutting out. - Suffixes commonly Suffixes commonly used are: ectomy--a cutting out (to cut out, excise, or excision) exeresis--removal (to strip out) 3. Amputation is cutting off. No suffix is used. these words mean amputation: However, Disarticulation Dismemberment 4. Introduction. introduction: No suffix is used. However, these words mean Injections Insertions Implantations Transfusions 5. Endoscopy is viewing or looking within. - The suffix used is: scopy (view) In this procedure a scope is used. procedure are: Some examples of this Anoscopy--viewing of the anus Bronchoscopy--viewing of bronchus Cystoscopy--viewing of the urinary bladder Otoscopy--viewing of the external ear 6. Repair means to form. Suffixes commonly used are: - plasty (to form) ostomy (a mouth) desis (a binding) pexy (a fixing) Procedures are as follows: plasty (repair or reform) (lengthen or shorten) (graft) (attach or reattach) (advancement) (recessions) (open reduction) ostomy(join together and form permanent openings between two distinct spaces.) desis(fusion) (stabilization) Example: Arthrodesis--a surgical stabilization of a joint. pexy (fixation) (suspension) 7. Destruction is a breaking down. - Suffixes commonly used are: clasis (destroy) (to break down) tripsy (crush) lysis (to loosen) (to free) Some procedures for which no suffixes are used but are of the destructive type are: cauterization (a branding iron) debridement (clean out) diathermy (heating through) fulguration (lightening) 8. Suturing is a sewing. - The suffix used is: rrhaphy (a seam) Some examples of this procedure are: Laryngorrhapy--suturing of larynx Arteriorrhaphy--suturing of artery Bronchorrhaphy--suturing of bronchus Enterorrhaphy--suturing of intestine 9. Manipulation is handling. - Suffixes commonly used are: tasis (a stretching) ectasia (out--a stretched condition or dilatation) Some procedures are: Closed reduction (of fracture) Application (of cast) II. CLASSIFICATION OF OPERATIONS AND NON-OPERATIVE PROCEDURES, FOR HIS ICD9-CM3 contains a detailed classification and an index of surgical operations and other therapeutic and diagnostic procedures, constructed to be useful within hospitals for hospital record-keeping. The Health Interview Survey uses only the first two digits of this classification. HIS recognizes that the detail about the specific type of operation is lost. It is felt that, for HIS, the body system or region (on which the operation was performed) will be sufficient for data analyses. As does ICD9-CM3, HIS recognizes 16 broad groups, each of which is subdivided into several 2-digit categories. These subdivisions describe operations within a system in one or more of the following ways: a. a specified operation with the part of body specified or implied, as in "excision of larynx" (30). b. operations without reference to procedures, for some conditions which are commonly surgical conditions, as in "operations for appendix" (47). c. operations, except as in a or b above (without reference to procedure or condition) on a specified part of body, as in "operations on esophagus" (42). Additionally, HIS has a single code category "00" for any operation of ill-defined or unknown type, with site unspecified. For the alphabetical arrangement of operations, or to determine the "broad" category for inclusion of a specific operation or procedure, see Index of Operations and Non-Operative Procedures (this Manual) or refer to ICD9-CM3. The ICD9-CM3 index can also be used for the classification of operations and procedures. Remember, however, that HIS only uses the first two digits. 1. OPERATION, TYPE UNKNOWN, SITE UNKNOWN 00 2. Operation, no site or type Includes: operation NEC on gland NOS, or "side," or other very ill-defined site. Use 00 when it is known that an operation was performed, but there is no information about the kind of operation or about the body system or part involved. OPERATIONS ON THE NERVOUS SYSTEM (01-05) 01 Incision and excision of skull, brain, and cerebral meninges 02 Other operations on skull, brain, and cerebral meninges 03 Operations on spinal cord and spinal canal structures 04 Operations on cranial and peripheral nerves 05 3. 4. 5. 6. Operations on sympathetic nerves or ganglia OPERATIONS ON THE ENDOCRINE SYSTEM (06-07) 06 Operations on thyroid and parathyroid glands 07 Operations on other endocrine glands OPERATIONS ON THE EYE (08-16) 08 Operations on eyelids 09 Operations on lacrimal system (tear ducts) 10 Operations on conjunctiva 11 Operations on cornea 12 Operations on iris, ciliary body, sclera, and anterior chamber 13 Operations on lens 14 Operations on retina, choroid, vitreous, and posterior chamber 15 Operations on extraocular muscles 16 Operations on orbit and eyeball OPERATIONS ON THE EAR (18-20) 18 Operations on external ear 19 Reconstructive operations on middle ear 20 Other operations on middle and inner ear OPERATIONS ON THE NOSE, MOUTH AND PHARYNX (21-29) 21 Operations on the nose Includes: operations on bone and skin of nose 22 Operations on nasal sinuses 23 Removal and restoration of teeth 24 Other operations on teeth, gums and alveoli 25 Operations on tongue 7. 8. 9. 10. 26 Operations on salivary glands and ducts 27 Other operations on mouth and face Includes: operations on lips, palate and soft tissue of face and mouth, except tongue and gingiva. 28 Operations on tonsils and adenoids 29 Operations on pharynx OPERATIONS ON THE RESPIRATORY SYSTEM (30-34) 30 Excision of larynx 31 Other operations on larynx and trachea 32 Excision of lung and bronchus 33 Other operations on lung and bronchus 34 Operations on chest wall, pleura, mediastinum and diaphragm OPERATIONS ON THE CARDIOVASCULAR SYSTEM (35-39) 35 Operations on valves and septa of heart 36 Operations on vessels of heart 37 Other operations on heart and pericardium 38 Incision, excision and occlusion of vessels 39 Other operations on vessels OPERATIONS ON THE HEMIC AND LYMPHATIC SYSTEM (40-41) 40 Operations in lymphatic system 41 Operations on bone marrow and spleen OPERATIONS ON THE DIGESTIVE SYSTEM (42-54) 42 Operations on the esophagus 43 Incision and excision of stomach 44 Other operations on stomach 11. 12. 45 Incision, excision, and anastomosis of intestine 46 Other operations on intestine 47 Operations on appendix 48 Operations on rectum and perirectal tissue 49 Operations on anus 50 Operations on liver 51 Operations on gallbladder and biliary tract Includes: operations on common bile duct, cystic duct and hepatic duct 52 Operations on pancreas 53 Repair of hernia 54 Other operations on abdominal region Includes: operations on epogastric region, flank, groin region, inguinal region, loin region, male pelvic cavity, mesentery, omentum, peritoneum and retroperitoneal tissue space. OPERATIONS ON THE URINARY SYSTEM (55-59) 55 Operations on kidney Includes: operations on renal pelvis 56 Operations on ureter 57 Operations on urinary bladder 58 Operations on urethra 59 Other operations on urinary tract OPERATIONS ON MALE GENITAL ORGANS (60-64) 60 Operations on prostate and seminal vesicles 61 Operations on scrotum and tunica vaginalis 62 Operations on testes 63 Operations on spermatic cord, epididymis, and vas deferens 64 Operations on penis 13. 14 15. OPERATIONS ON THE FEMALE GENITAL ORGANS (65-71) 65 Operations on ovary 66 Operations on fallopian tubes 67 Operations on cervix 68 Other incision and excision of uterus 69 Other operations on uterus and supporting structures 70 Operations on vagina and cul-de-sac 71 Operations on vulva and perineum OBSTETRICAL PROCEDURES (72-75) 72 Forceps, vacuum, and breech delivery 73 Other procedures inducing or assisting delivery Includes: spontaneous delivery with manual assistance. 74 Caesarean section and removal of fetus 75 Other obstetric operations For HIS, includes also "normal delivery" OPERATIONS ON THE MUSCULOSKELETAL SYSTEM (76-84) 76 Operations on facial bones and joints Excludes: accessory sinuses (22); nasal bones (21); and skull (01) (02). 77 Incision, excision and division of other bones Excludes: operations on facial bones (76); joint structures (80-81); nasal bones (21); and skull (01- 78 Other operations on bones, except facial bones Excludes: operations on joint structures (80-81); nasal bones (21); and skull (01-02). 79 Reduction of fracture and dislocation Includes: application of cast or splint; reduction with insertion of traction device. Excludes: external fixation alone for immobilization of fracture (93); internal fixation without reduction of fracture (78); operations on facial bones (76); nasal bones (21); orbit (76); skull (02); vertebrae (03); removal of cast or splint (97); replacement of 02). cast or splint (97); and traction alone for reduction of fracture (93). 16. 80 Incision and excision of joint structures Includes: operations on capsule of joint; cartilage; condyle; ligament; meniscus; and synovial membrane. Excludes: cartilage of ear (18); nose (21); and joints of face (76) 81 Repair and plastic operations on joint structures Includes: operations on ankle; knee; shoulder; elbow; wrist; hip; vertebrae; fingers; and toes not codable elsewhere and when condition is not specified and tissue (such as skin, bone, etc.) is not apparent. 82 Operations on muscle, tendon and fascia of hand Includes: operations on synovial membrane and tendon sheath. 83 Operations on muscle, tendon, fascia and bursa, except hand Includes: operations on synovial membrane and tendon sheaths. Excludes: hand (82); diaphragm (34); and muscles of eye (15). 84 Other procedures on musculoskeletal system OPERATIONS ON THE INTEGUMENTARY SYSTEM (85-86) 85 Operations on the breast Includes: operations on skin of breast and previous mastectomy site (male or female). 86 Operations on skin and subcutaneous tissue Includes: operations on hair follicles; male perineum; nails; sebaceous glands, subcutaneous fat pads; and superficial fossae. Includes also: operations on regions such as arm; armpit; back; cheek; buttock; chin; feet; forearm; forehead; hand; head; heel; leg; neck; scalp; temple; temporal region; and thigh when specific tissue (such as bone; cartilage; joint; etc.) involved is not mentioned. Excludes: those on skin of anus (49); breast (85); ear (18); eyebrow (08); eyelid (08); female perineum (71); lips (27); nose (21); penis (64); scrotum (61); and vulva (71). Excludes also: operations on the head when a specific part (such as brain) is mentioned. 17. MISCELLANEOUS DIAGNOSTIC AND THERAPEUTIC PROCEDURES (87-99) 87 Diagnostic radiology Includes: diagnostic X-Rays of face, head, neck, brain, skull, spine, chest, digestive tract, urinary system, genital organs, abdomen and breast. Excludes: angiography (88). 88 Other diagnostic radiology and related techniques Includes: CAT scan of abdomen; skeletal X-Rays of extremities; arteriograms using contrast material; and X-Rays of veins Excludes: angiogram of eye (95). 89 Interview, evaluation, consultation, and examination Includes: diagnostic interviews; neurological exams; gynecological exams; dental exams; digital exams of rectum; pacemaker checks; electrocardiograms and general physical exams. 90 Microscope examination-I Includes: microscopic examination of specimen from nervous system, spinal fluid, endocrine glands, eye, ear, nose, throat, larynx, trachea, bronchus, pleura, lung, of sputum, and blood. 91 Microscope examination-II Includes: microscope examination of specimen from liver, bile ducts or passage, pancreas, peritoneal, retroperitoneal, kidney, ureter, perirenal, periureteral tissue, bladder, urethra, prostate, seminal vesicle, urine, semen, and female genital tract. 92 Nuclear medicine Includes: scans, radiation therapy (cobalt) (iodine) 93 Physical therapy, respiratory therapy, rehabilitation, and related procedures Includes: diagnostic physical therapy; physical therapy exercises such as breathing exercises; manual traction; training in use of prosthesis; whirlpool treatments; heat therapy; skeletal traction; speech and reading training for blind; play therapy; education therapy; artificial respiration, mouth to mouth resuscitation; oxygen and helium therapy. 94 Psychiatric interviews, consultations and evaluationsIncludes: routine psychiatric visits; psychoanalysis; psychiatric electroshock therapy and other nonoperative procedures relating to the psyche. 95 Ophthalmologic and otologic diagnosis and treatment Includes: eye exams, functional tests of eye; fitting glasses, contact lens; hearing tests; and fitting hearing aids. 96 Non-operative intubation and irrigation Includes: insertion of airway tubes; ear packing; vaginal packing; insertion of vaginal diaphragm; rectal packing; dilation of rectum and anus; manual reduction of hernia; gastric lavage; irrigation of ostomies; cleaning of eye, ear and nose; lavage of bronchus and trachea. 97 Replacement and removal of therapeutic appliances Includes: replacement of ostomy tubes; replacement of casts; removal of nasal packing, removal of dental wiring; removal of sutures from head and neck; removal of drainage tubes; removal of IUD and removal of sutures from genital tract. 98 Non-operative removal of foreign body Includes: removal of foreign body from digestive system, ear, nose, larynx, vagina, etc., WITHOUT INCISION. 99 Other non-operative procedures Includes: blood transfusion, exchange transfusions, injections or infusions of therapeutic or prophylactic substances; cardiac pacemaker (battery and sensing types), acupuncture and fitting of dentures. X-CODES FOR SPECIAL IMPAIRMENTS (X00-X99) BY TYPE, SITE AND ETIOLOGY NATIONAL HEALTH INTERVIEW SURVEY ICD-9 REVISION (NCHS, 1979): X-CODES U. S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service National Center for Health Statistics Division of Health Interview Statistics I. History and Development of the X-Code The X-Code for Special Impairments, by Type, Site and Etiology, was developed in 1955-1956 by the, at that time, Division of Public Health Methods of the Public Health Service. It had been tried and found useful in coding and tabulating various kinds of defects and deformities as reported in household health surveys, and in certain other studies of handicapping conditions, requiring relatively simple detail for statistical presentation. It provided a plan by which all three elements of type, site, and etiology (cause) could be expressed by means of a single diagnostic code, and it brought together similar types of defects by type and site; etiology was supplied by adding an additional 1-digit code to the code for the type and site. HIS elected to use this plan for impairments, using the ICD revision in effect at that time for coding all other conditions. HIS had continued to use this X-Code, making some changes in it since the beginning of the Survey, and will use it instead of the present ICD for the coding of impairments. Part III of this section, which contains the X-Code and explains it, has been rewritten as of January 2, 1979 but the methods are similar to what they were before the lists of etiological codes, and the full of impairments by type, see V and VI of this principles and that time. For classification section. In developing the X-Code it had been necessary to make a selection of conditions to be called "impairments." The term "impairment" has no actual definitive medical significance. Cardiac, mental, or arthritic patients are "impaired" as well as amputees, the blind, the deaf. However, defects of the heart, lungs, other internal or respiratory organs are in general excluded from the X-Code except when these sites are involved in paralysis, absence of part, or postoperatively in the formation of an artificial opening to the surface. Chronic progressive disease processes of all systems are excluded (to be coded as per ICD), but the line between what is a "chronic disease" and what is an "impairment" is, in some areas, admittedly thin. For example: speech defects, mental retardation, cerebral palsy are included in the XCode, but epilepsy, multiple sclerosis, Parkinson's disease, and personality defects are to be coded as chronic diseases in terms of ICD codes. II. General Characteristics of Special Impairments 1. Special impairments (to be referred to hereinafter as impairments) are often late effects of past and inactive pathological processes. (See also Item D, following, for discussion of "late effects.") But they may sometimes coexist with and be due to a currently active progressive chronic disease such as diabetes, arteriosclerosis, cancer. 2. Impairments are often, but by no means necessarily, permanent, and some are relatively minor in nature. Many respond to corrective therapy, medical or surgical. However, they must be chronic or long-continuing or of chronic type in order to be coded to the X-Code. For rules for coding impairments in relation to date of onset, see specific instructions set forth elsewhere in the HIS Medical Coding Manual. III. Table of X-Codes for Special Impairments ICD CODE NUMBER(S) NAME OF IMPAIRMENT X-CODE NUMBERS 317-319 Mental Retardation (various degrees) X19 342 X41 Hemiplegia 343 Infantile cerebral palsy X50 Other paralytic syndromes For 344.0-344.9: X40-X49, X50-X59, X60-X63 by site and extent 344.0 344.2 344.3 344.4 344.5 344.6 344.8 344.9 Quadriplegia Diplegia of upper limbs Monoplegia of lower limbs Monoplegia of upper limbs Unspecified monoplegia Cauda equina syndrome Other Unspecified 369 Blindness and low vision X00-X03 389 Deafness Deaf mutism X05-X09 X05 520.0 520.1 520.2 520.5 Anodontia Supernumerary teeth Abnormalities of size and form Hereditary disturbances, tooth structure X92 X92 X92 521.6 Ankylosis, teeth X92 524 Dentofacial anomalies, including malocclusion X92 718.6 Unspecified Protrusio Acetabuli X75 728.4 Laxity of ligament X80-X89, by site 728.6 Contracture of palmar fascia X74 734 Flatfoot X77 735 Acquired deformities of toe X76 736 Other acquired deformities of limbs X73-X76, by site X92 737 Curvature of spine 738 738.0 738.1 738.2 738.3 738.4 738.5 Other acquired deformities Acquired deformity of nose Other acquired deformity of head Acquired deformity of neck Acquired deformity of chest and rib Acquired spondylolisthesis Other acquired deformity of back and spine Acquired deformity of pelvis Cauliflower ear Acquired deformity of other specified site 738.6 738.7 738.8 738.9 X70 X90 X93 X79 X79 X70 X70 X75 X90 X70-X79, X90, X92, X93 by site Acquired deformity of unspecified site X99 741 Spina bifida (congenital) X71 742.0 742.1 742.3 Encephalocele Microcephalus Congenital hydrocephalus X93 X93 X93 743.0 Anophthalmos, one or both eyes 743.1 Microphthalmos, NEC X00, X02, X03 X01 744.0 Anomalies of ear, causing hearing impairment X05-X09 Accessory auricle Other specified anomalies of ear Unspecified anomalies of ear Webbing of neck X90 X90 X90 X79 Other specified anomalies of: face (disfiguring) NEC neck NEC X90 X79 Unspecified anomalies of: face NEC neck NEC X90 X79 749 Cleft palate and cleft lip X91 750.0 750.1 Tongue tie Other anomalies of tongue (with speech defect) X11 X11 Renal agenesis and dysgenesis X31 744.1 744.2 744.3 744.5 744.8 744.9 753.0 Congenital musculoskeletal 754.2 754.3 754.4 754.5 754.6 754.7 754.8 755 deformities: spine dislocation of hip bowleg(s), deformity leg(s) various deformities of feet valgus deformities of feet: flatfoot other deformities of feet: clubfoot NOS other specified deformities: chest wall dislocation of elbow chest, funnel chest clubhand, hand lower limb joints Other congenital anomalies of limbs: absence X70 X75 X76 X76 X76 X77 X76 X78 X79 X73 X79 X74 X76 X20-X29, X35, by site X70-X79, deformity by site 756.0 756.1 756.2 756.3 Anomalies of skull and face bones: face skull, NEC Anomalies of spine: absence of specified segment of spine other deformity of spine Cervical rib Other anomalies of rib and sternum: absence of rib, sternum other deformity of rib and sternum 758.0 Down's syndrome 759.7 759.9 781.5 Multiple congenital anomalies, type and site not specified Clubbing of fingers Traumatic amputation of: thumb(s) other finger(s) arm(s) and hand(s) 885 886 887 895 896 897 905.6 905.7 905.8 905.9 effect effect effect effect of of of of X33 X70 X79 X33 X79 X19 toe(s) feet, foot leg, one or both Late Late Late Late X90 X93 dislocation sprain and strain tendon injury traumatic amputation X99 X74 X22, X25 X22, X25 X20-X24 X27, X29 X27, X29 X26, X28 X80-X89 X80-X89 X80-X89 X20-X29, X35 IV. Late Effects of Diseases, Injuries, and Poisonings A "late effect" is regarded generally as any abnormal condition resulting from a pathological process after this causative process has become inactive or healed. A late effect may consist of an impairment as defined, or it could be any other abnormal condition. For HIS, an impairment may be a late effect as defined above, but it could be due to some active pathological process. By means of the X-Code, impairments can be collected whether the cause is present or not. Instructions for coding impairments in relation to active diseases causing them are set forth elsewhere in the HIS Medical Coding Manual. For facts about the HIS method of coding late effects of injury or poisoning, see notes for categories 800-999, in Appendix III. ICD has a few categories specific for late effects of certain diseases--with "late effects" in the title. Following are the ICD codes for late effects of diseases, which are not used by HIS for any purpose--as shown also in Appendix III: 137 138 139 268.1 326 438 Late effects of tuberculosis Late effects of acute poliomyelitis Late effects of other infectious and parasitic diseases Late effects of rickets Late effects of intracranial abscess or pyogenic infection Late effects of cerebrovascular disease Since the above ICD late effects codes for diseases are not used by HIS, the method for coding late effects is as follows: 1. When the late effect is a specified impairment, the appropriate X-Code is selected and the appropriate etiological code is added; for example, "Blindness, both eyes, due to old trachoma" is coded by HIS to X00.8, only. 2. When the late effect is specified but it is not an impairment, the condition is coded to the ICD code for the condition, but never to the above-mentioned ICD late effects codes; for example, "Personality disorder due to old encephalitis" is coded by HIS to 301.9 only. 3. If the past disease is known, but the present difficulty is ill-defined or not specified, the above ICD late effects codes will not be used. Instead, if the past disease is one indicated in these late effects codes, or is an old birth injury, or "Brain damage NEC," X99 (with the appropriate etiological code) will be used. Thus, for example, "Postpolio" would be coded to X99.4. However, for "After effects of stroke," the stroke only will be coded. Coding Late Effects, other than those on the preceding page, and the original disease. 1. ICD does not have late effect codes for diseases other than the ones mentioned on preceding page. However, HIS coding requires that late effects of many diseases be coded; which is usually a special impairment. When the disease causing the late effect or special impairment is given, the question arises as to when to code the causative disease also. HIS has devised a definition for an active disease and an inactive disease, as described below. When a causative disease meets HIS' definition of "active" disease, the disease will be coded in addition to any special impairment. Inactive disease processes will be used to determine etiology and will not be coded as a separate diagnosis. The terms "active" and "inactive" (i.e., past disease or condition) are distinguished by considering ALL of the Condition Pages for each person. The CAUSE is "Active" if: 1. it is reported in Item 1 or 3b of a Condition Page AND NO explicit indication that the condition is cured or no longer present. "Explicit indication" would include: (1) Questions 10-12 completed and "cured" is checked in Item 12b; (2) the person says the condition is cured, corrected, arrested, no longer present, or the equivalent; (3) the name of the condition indicates it is no longer present ("childhood rheumatic fever" and the person is an adult) 2. it is reported in Item 3c or 3e, and the condition is on the list of conditions which are coded as chronic regardless of onset, and there is no explicit indication (as defined in #1 above) that the condition is cured, corrected, arrested or no longer present 3. it is stated in the footnotes or written entries of the Condition Page(s) for this person that the disease causing the impairment is still present. The CAUSE is "Inactive" if: 1. it is reported in Item 1 or 3b if a Condition Page AND there is explicit indications (as defined in #1 above) that the condition is cured, corrected, arrested or no longer present 2. it is reported in Item 3c or 3e, AND either the condition is NOT on the list of conditions which are coded as chronic regardless of onset, or, if it is on the list, there is explicit indication (as defined in #1 above) that the condition is cured or no longer present 3. there is no footnote or written entry on the Condition Pages for this person stating that the condition causing the impairment is still present. V. List of Etiology Codes Note: For complete instruction for coding impairments by etiology, see HIS Medical Coding Manual. These codes are listed in the order of priority in which they should be assigned when more than one etiology is given, none of them is an accident or injury, and it cannot be determined which was the earlier cause of impairment. Where the earlier cause can be determined, that fourth digit is always assigned regardless of the priority shown below. Accident or injury ALWAYS has priority whether it occurred earlier or later than other causes. .0 Accident or injury except at birth .1 Cerebrovascular disease (stroke) (with arteriosclerosis) (with hypertension) .2 Neoplasm .3 Diabetes (with cataract or glaucoma) .4 Poliomyelitis .5 Cataract with glaucoma .6 Cataract without glaucoma .7 Glaucoma without cataract .8 Other eye diseases (as in ICD) (any infection of eye) .9 Congenital origin NEC or birth injury .X Other conditions not in .0-.9 (non-congenital) (nontraumatic) (heredity) (old age) ("age" NOS) .Y Unknown or unspecified origin VI. Classification of Impairments, by Type and Site (X00-X99) Note: For complete instructions for coding all types of impairments and their causes, see HIS Medical Coding Manual, Section V. X00-X03 BLINDNESS AND IMPAIRMENT OF VISION (Only one code in this range may be used per person) X00 Blind in both eyes X01 Visual impairment in both eyes X02 Blind in one eye, visually impaired in the other eye X03 Blind or visually impaired in one eye only; other eye, good vision or not mentioned X05-X09 DEAFNESS AND IMPAIRMENT OF HEARING (Only one code in this range may be used per person.) X05 Deafness, both ears Includes persons who are reported "deaf" in both ears, or reported to have no useful hearing in both ears, or can't hear, both ears. Excludes terms such as "a little deaf," "partially deaf"; code as for hearing impairment. X06 Other hearing impairment involving both ears Any bilateral hearing impairment which cannot be coded to X05. X07 Deafness or hearing impairment of any degree involving only one ear X08 Deafness, NOS Includes "deafness," "deaf," "can't hear," unknown whether one or both ears are involved. Excludes such terms as "a little deaf," "partially deaf"; code as for hearing impairment. X09 Impaired hearing, NOS Hearing impairment unknown whether one or both ears are involved. X10-X19 IMPAIRMENT OF SPEECH, SPECIAL SENSE AND INTELLIGENCE X10-X11 IMPAIRMENT OF SPEECH (Only one code in this range may be used per person.) X10 Stammering and Stuttering X11 Other speech defects Includes persons with absence of larynx or tongue even when speech defect is not reported. X12 X12 X14 IMPAIRMENT OF SPECIAL SENSE, EXCEPT VISION OR HEARING Loss or impairment of sensation Includes taste, smell and loss or disturbances of sensation (burning) and numbness of any body parts. This code will NOT be used if a code in the range of X40-X99 had been assigned. SPECIAL LEARNING DISABILITY (Prefer X19 if both X14 and X19 are present per person) X14 X19 X19 Special learning disability (reading) (mathematics) ("mirror writing or reading".) Does not include learning disability resulting only from deficiency in intelligence. MENTAL RETARDATION Mental retardation: Any degree or any type, including "mongolism" ("Down's (Downes) Syndrome") X20-X35 ABSENCE, LOSS, EXTREMITIES, AND CERTAIN OTHER SITES X20-X25 ABSENCE, LOSS, UPPER EXTREMITY (For X20-X22, code only one of these codes per person, and no other code in the range of X20-X25.) X20 Arms, both X21 Hands, both X22 One or more fingers (excludes tip only-below first joint (thumb(s)) of both hands X23 Arm, one Person may also have X24 or X25 X24 Hand, one Person may also have X23 or X25 X25 One or more fingers, (excludes tip only-below first joint), thumb, of only one hand. Person may also have X23 or X24. X26-X29 ABSENCE, LOSS, LOWER EXTREMITY (For X26-X27, code only one of these codes per person, and no other code in the range of X26-X29.) X26 Legs, both X27 Feet or toes (excludes tip only-below first joint) only, both X28 Leg, one Person may also have X29 X29 Foot or toes (excludes tip only-below first joint) only, one Person may also have X28 X30-X35 ABSENCE, LOSS, CERTAIN OTHER SITES X30 Lung X31 Kidney X32 Breast X33 Rib, bone, joint, or muscle of trunk, one or more X34 Bone, joint, or muscle of extremity (including hip) without loss of extremity, one or more X35 Tips of fingers or toes (below first joint) only X40-X64 PARALYSIS, COMPLETE OR PARTIAL X40-X49 PARALYSIS NOS (COMPLETE) OF EXTREMITIES X40 Entire body or four limbs (Prohibits X41-X62) X41 One side of body only, including limbs; or "hemiplegia." (Prohibits X42, X44, X46, or X48) X42 Arms, both X43 Arm, one (Prohibits X44) (When the corresponding body part on the other side has been coded, "one" means "other.") X44 Hands, both, and/or finger(s) (thumb) on one or both hands only. (Prohibits X45) X45 Hand, one, and/or finger(s) (thumb) on one hand, only. (When the corresponding body part on the other side has been coded, "one" means "other.") (Prohibits X43-X45) X46 Legs, both; or "paraplegia." X47 Leg, one (Prohibits X48) (When the corresponding body part on the other side has been coded, "one" means "other.") X48 Feet, both, and/or toe(s) on one or both feet, only. (Prohibits X49) X49 Foot, one, and/or toe(s) on one foot, only. (When the corresponding body part on the other side has been coded, "one" means "other.") X50-X59 (Prohibits X47-X49) CEREBRAL PALSY AND PARALYSIS PARTIAL OF EXTREMITIES X50 Cerebral palsy (and synonyms) Includes "spastic" if present since birth (congenital) (Prohibits X51-X64) X51 One side of body only, including limbs; or "hemiparesis." (Prohibits X52, X54, X56, or X58) X52 Arms, both X53 Arms, one (Prohibits X54) (When the corresponding body part on the other side has been coded, "one" means "other.") X54 Hands, both, and/or finger(s) (thumb) on one or both hands only. (Prohibits X55) X55 Hand, one, and/or finger(s) (thumb) on one hand only. (When the corresponding body part on the other side has been coded, "one" means "other.") X56 Legs, both; or "paraparesis" X57 Leg, one (Prohibits X58) (When the corresponding body part on the other side has been coded, "one" means "other.") X58 Feet, both, and/or toe(s) on one or both feet, only (Prohibits X59) X59 Foot, one, and/or toe(s) on one foot, only (When the corresponding body part on the other side has been coded, "one" means "other.") X60-X64 X60 (Prohibits X53-X55) (Prohibits X57-X59) PARALYSIS, COMPLETE OR PARTIAL, SITES EXCEPT EXTREMITIES Trunk, any part except parts included in X40, X41, or X51. If PARTIAL paralysis of ENTIRE body is indicated, code X60 only. X61 Face (Bell's palsy or paralysis) X62 Bladder or anal sphincter X63 Paralysis, complete or partial, sites NOT of extremities, trunk, nor affecting special senses or speech X64 Paralysis, complete or partial, NEC X70-X79 Includes: SPECIFIED DEFORMITY OF LIMBS, TRUNK, BACK specified structural deformities of limbs, trunk, back, described as: contracture; atrophy; accessory ("extra"); short or shortness; crippled; shrivelled; "drawn up"; "twisted"; "withered"; and scarring (with contracture) involving limbs, neck, back, trunk. X70 Curvature and other structural deformities of spine or back, except as in X71.9 Includes: all structural deformities of spine or back except spina bifida (X71.9) Excludes: chronic back conditions NEC in X80 X71.9 Spina bifida (with meningocele) (always congenital) Excludes: X80 X73 Deformity of shoulder or upper extremity Excludes: deformity of hand(s), finger(s), thumb(s), only (Prohibits X74) X74 Deformity of hand(s), finger(s), thumb(s), only X75 Dislocation, congenital, and other deformity hip and/or pelvis X76 Deformity of any site on lower extremity, one or both Includes: genu valgum (knock knee); genu varum (bowleg); tibial torsion; hammer toe; hallux valgus or varus; any deformity of toe; deformity leg NOS, foot NEC, knee. Exludes X77 and X78. X77 Flatfoot (including weak or fallen arches and other difficulty with arches) X78 Clubfoot (congenital) X79 Deformity, neck, trunk bones, NEC Includes: pigeon breast; cervical rib; postural defect NEC X80-X89 NON-PARALYTIC ORTHOPEDIC IMPAIRMENT (CHRONIC) NEC Excludes: "disc conditions" (ICD 722) Includes: Limitation of motion NEC; stiffness (complete or partial); "flail joint"; instability of joint; symptomatic, but chronic difficulty, weakness, "trouble", pain, swelling, "limping," involving muscles, joints, limbs, back or trunk, of unknown cause or due to healed injuries 3 months + or to past and now inactive diseases; old (3 months +) sprains, strains, or dislocations with effect not elsewhere classifiable, or not stated. Note: Orthopedic impairment NEC, as in X80-X89, is not to be coded as a separate diagnosis if due to specified disease; code disease only. ORTHOPEDIC IMPAIRMENT NEC (CHRONIC) INVOLVING: X80 Back, any part Includes: neck X84 Shoulder(s) and/or upper extremity(ies) X85 Hip and/or pelvis Excludes: Congenital dislocation of hip (X75.9) X86 Lower extremity Excludes: impairments involving arches of foot, feet (X77) X89 Other and ill-defined sites Includes: rib; trunk, NOS; "side," NOS; joint, NOS; limping; staggering; stumbling; trouble in walking, NOS Excludes: jaw (X92); and ataxic gait, which if chronic, is coded as for Paralysis, partial. X90-X99 DEFECT, ABNORMALITY, SPECIAL IMPAIRMENT, NEC X90 Disfigurement, scarring, face, nose, lips, ears Includes: absence of nose, lips, ears; accessory auricle; other abnormality NEC of face, nose, ears, mouth, teeth, jaws if stated to be disfiguring. If speech defect is present, code it also. Excludes: cleft palate and harelip whether or not disfiguring (X91.9.) X91.9 Cleft palate and harelip (with speech defect) (disfiguring) Includes: cleft palate and cleft lip (as in ICD 749) with or without speech defect and whether or not stated to be disfiguring. X92 Other dentofacial handicap Includes: acquired absence of teeth, onset 3 months +; and abnormalities of teeth, malocclusion, and other jaw and dentofacial anomalies as in ICD 520.0, 520.1, 520.2, 520.5, 521.6 and 524. If speech defect is present, code it also. Excludes: cleft palate and harelip (X91.9); and other dentofacial handicaps if stated to be disfiguring (X90). X93 Deformity of skull (hydrocephaly) (microcephaly). mental retardation is present, code it also. X94 Artificial orifice (opening) or valve (surgical) any site (colostomy) X99 Special impairment, ill-defined Includes: deformed NOS; cripple NOS; "birth injury" or "brain damage" NOS, at ages 3 months or over without specification as to type of impairment; ill-defined "after effects" of tuberculosis, encephalitis, poliomyelitis, trachoma, toxoplasmosis, other infective and parasitic diseases, rickets, intracranial abscess. See also Item D, this Appendix. Excludes: stroke, or ill-defined "after effects" of stroke; code the stroke--not X99.Section Q If MEDICINE NAMES AND CODES The system adopted for processing prescribed medicine data in NMES was first developed as part of the 1980 National Ambulatory Medical Care Survey (NAMCS) by the National Center for Health Statistics (Koch 1980). 99999 99980 00010 00095 00100 00105 00110 60000 00140 00163 00210 00220 00223 ILLEGIBLE OTHER A AND D A.P.L. A.S.A. A.S.A. & CODEINE A.S.A. COMPOUND A.S.A. ENSEAL A-FIL A/T/S ACCELERASE ACCURBRON ACCUTANE 00245 00260 00265 00270 00275 00280 00283 00288 00295 00305 00320 00340 60030 00345 00355 60035 00395 00455 00475 00481 00480 00487 60040 00510 00525 60045 00540 60050 00560 00570 00585 60060 00590 60065 00595 00597 00598 00615 00645 41870 41825 00685 00705 00735 00740 00790 00805 00810 00825 00830 00835 00845 00850 00880 ACETA ACETAMINOPHEN ACETAMINOPHEN NO. 2 ACETAMINOPHEN NO. 3 ACETAMINOPHEN NO. 4 ACETAMINOPHEN W/CODEINE ACETAMINOPHEN W/OXYCODONE ACETASOL ACETAZOLAMIDE ACETIC ACID ACETONE ACHROMYCIN ACHROMYCIN OPHTHALMIC ACHROMYCIN V ACI-JEL ACLOVATE ACNE ACTH ACTIFED ACTIFED WITH CODEINE ACTIFED-C ACTRAPID ADALAT ADAPIN ADEFLOR ADEFLOR M ADIPEX ADIPEX-P ADRENALIN ADRIAMYCIN ADSORBOCARPINE ADSORBOCARPINE OPHTHALMIC ADSORBONAC ADSORBONAC OPHTHALMIC ADSORBOTEAR ADVIL AEROBID AEROSEB-HC AFRIN AK-SPORIN AKARPINE AKINETON AL-AY ALBALON ALBALON-A ALCOHOL ALCOHOL RUBBING ALCON ALDACTAZIDE ALDACTONE ALDOCLOR ALDOMET ALDORIL ALKA-SELTZER 00905 00915 00925 00935 00960 00975 00976 00979 00980 00990 01030 60070 01100 01145 01155 01221 01225 01235 01250 01255 01258 01290 01305 60075 01315 01325 01335 01375 01395 01410 01420 01450 01525 01530 01535 01615 01630 01635 01640 01660 01670 01685 01690 01700 01725 01730 01755 01758 01775 01780 01820 01838 01840 01850 ALKALOL ALKERAN ALLBEE ALLBEE W/C ALLEREST ALLERFRIN ALLERGAN ALLERGINE ALLERGY RELIEF OR SHOTS ALLERNADE T.D. IMPROVED ALLOPURINOL ALPHA-TREX ALPHADERM ALTERNAGEL ALU-CAP ALUMINUM ACETATE ALUMINUM HYDROXIDE ALUMINUM MAGNESIUM HYDROXIDE ALUMINUM-MAGNESIUM HYDROX W/SIMETHICO ALUPENT ALUPRIN AMANTADINE AMBENYL AMBENYL EXPECTORANT AMCILL AMEN AMERICAINE AMICAR AMIDOXINE AMIKIN AMINO-CERV AMINOPHYLLINE AMITRIL AMITRIPTYLINE AMITRIPTYLINE HCL W/PERPHENAZINE AMOLIN AMOXICILLIN AMOXICILLIN TRIHYDRATE AMOXIL AMPHETAMINE AMPHOJEL AMPICILLIN AMPICILLIN TRIHYDRATE AMQUINTUSSIN AMYTAL ANA EMERGENCY INSECT STING KIT ANACIN ANACIN-3 WITH CODEINE ANALGESIC ANALGESIC BALM ANAMINE ANAPROX ANASPAZ ANATUSS 01853 01855 01860 01983 01995 01990 02050 02053 02070 02075 02080 02088 02113 02115 02120 02135 02150 02155 02125 02157 02158 02159 02165 35410 35415 35420 35425 35440 35445 35450 35455 35460 35465 02180 35475 35480 35483 35490 35495 02195 02205 02215 02220 02225 35505 02235 02250 35510 02285 02310 02315 02335 02340 02345 ANATUSS W/CODEINE ANAVAR ANBESOL ANESTHETIC ANEXSIA ANEXSIA W/CODEINE ANOQUAN ANOREXIC AGENT ANSPOR ANTABUSE ANTACID ANTACID AND ADSORBENT ANTHELMINTIC AGENT ANTHRA-DERM ANTHRALIN ANTI-ITCH ANTI-TUSS ANTI-TUSS DM ANTIACID ANTIANEMIA AGENT ANTIBIOTIC AGENT ANTIBIOTIC EAR DROPS ANTICOAGULANT ANTICONVULSANT AGENT ANTIDEPRESSANT AGENT ANTIDIABETIC AGENT ANTIDIARRHEAL AGENT ANTIFLATULENT AGENT ANTIFUNGAL AGENT ANTIHISTAMINE ANTIINFECTIVE AGENT ANTIINFLAMMATORY AGENT ANTILIPEMIC AGENT ANTIMINTH ANTINAUSEANT AGENT ANTINEOPLASTIC AGENT ANTIOBESITY AGENT ANTIPRURITIC AGENT ANTIPYRETIC AGENT ANTIPYRINE ANTISEPTIC MOUTHWASH ANTISEPTIC SOLUTION ANTISPAS ANTISPASMODIC ANTITUBERCULAR AGENT ANTIVENIN SPIDER BITE ANTIVERT ANTIVIRAL AGENT ANTURANE ANUSOL ANUSOL-HC APAP APAP W/CODEINE APAP W/CODEINE ELIXIR 02395 02405 02410 02445 02465 02473 02485 02510 02513 02520 02570 02575 02580 02605 02625 02627 02645 02660 02690 02705 02725 41980 02748 02805 02820 02803 02828 02835 02870 02875 02880 02900 02910 02925 02940 02950 02980 02982 60115 02987 60120 60125 60130 02995 03005 03020 03025 03040 03045 03050 03065 03070 03110 03113 APRESAZIDE APRESOLINE APRESOLINE-ESIDRIX AQUACARE AQUAMEPHYTON AQUAPHYLLIN AQUASOL A AQUATENSEN AQUEOUS BENZALKON CHLOR ARALEN ARISTO-PAK ARISTOCORT ARISTOCORT A ARISTOCORT R ARLIDIN ARMOUR THYROID ARTANE ARTHRITIS PAIN FORMULA ASBRON G ASCORBIC ACID ASCRIPTIN ASCRIPTIN A/D ASENDIN ASPIRIN ASPIRIN COMPOUND #3 ASPIRIN W/CODEINE ASPIRIN W/OXYCODONE ASPIRIN-PHENACETIN-CAFFEINE ATABRINE ATARAX ATARAXOID ATIVAN ATRIDINE ATROMID-S ATROPINE ATROPINE SULFATE ATROPISOL ATROVENT AUGMENTIN AUGMENTIN 125 AUGMENTIN 250 AUGMENTIN 500 AURAFAIR OTIC SOLUTION AURALGAN AUREOMYCIN AUROTO DROPS AVALGESIC LOTION AVC AVC/DIENESTROL AVEENO AVEENO-BAR AVENTYL HCL AXOTAL AYGESTIN 03123 03125 03130 40170 03135 03140 03205 03225 03250 03265 03275 03330 03263 03355 03365 03395 03410 03415 03420 03423 35530 03430 03435 03480 03485 03510 03515 03518 03520 03540 03560 03605 03635 40185 03700 03705 03709 03715 03743 03740 03741 03785 03800 03805 03825 03845 03850 03880 03885 03890 03900 03905 03920 03955 AZATHIOPRINE AZENE AZMA-AID AZMA-CORT AZO GANTANOL AZO GANTRISIN AZOLID AZULFIDINE B COMPLEX B COMPLEX FORTE B COMPLEX W/ASCORBIC ACID & B-12 B-C-BID B-COMPLEX C W/IRON B-12 BABY COUGH SYRUP BACID BACITRACIN BACITRACIN-NEOMYCIN-POLYMYXIN BACITRACIN-POLYMYXIN BACLOFEN BACTINE BACTRIM BACTRIM DS BALNEOL LOTION BALNETAR BANALG LINIMENT BANCAP BANCAP HC BANCAP W/CODEINE BANTHINE BARBIDONNA BAROPHEN ELIXIR BASALJEL BAYER ASPIRIN BECLOMETHASONE BECLOVENT BECONASE BECOTIN BEE STING ANTIVENIN BEELITH BEEPEN VK BELLADENAL BELLADONNA BELLADONNA ALKALOIDS W/PHENOBARBITAL BELLADONNA W/PHENOBARBITAL BELLERGAL BELLERGAL-S BEMEX BEMINAL BEMINAL FORTE W/VITAMIN C BEN GAY BENADRYL BENAHIST BENEMID 03970 03985 04000 04015 04020 04025 04030 60175 04055 04065 04085 04088 60180 04100 04105 04188 04120 04125 04135 60185 04160 04170 60200 04180 40205 04190 60205 40210 04225 04235 04240 04250 04295 04323 04355 04365 04370 04385 04425 04433 04480 04485 04511 04513 04515 04580 04585 04590 04595 50000 60235 04650 04660 04668 BENOQUIN BENSULFOID BENTYL BENYLIN SYRUP BENZAC BENZAC W GEL BENZAGEL BENZAMYCIN BENZOCAINE BENZODENT BENZOYL BENZOYL PEROXIDE BENZTROPINE MESYLATE BEROCCA BEROCCA-C BETA-VAL BETADINE BETADINE PERINEAL WASH CONCENTRATE BETADINE VAGINAL GEL BETAGAN LIQUIFILM BETALIN BETAMETHASONE BETAMETHASONE VALERATE BETAPEN-VK BETATREX BETHANECHOL BETOPTIC BI-K BICARBONATE OF SODA BICILLIN BICILLIN C-R BICITRA BILRON BIONATE BIOZYME BIPHETAMINE BISACODYL BISCODYL BISMUTH SUBGALLATE BISMUTH SUBGALLATE FORMULA W/HYDROCOR BLEPH BLEPHAMIDE BLOCADREN BLOOD FORMATION AND COAGULATION BLUBORO BONINE BONTRIL PDM BORAX BORIC ACID BRETHAIRE BRETHANCER BRETHINE BREVICON BREXIN 04670 04760 04770 04790 04803 04808 04845 04825 04873 04875 04895 04915 04920 04925 04930 04970 04975 05010 05015 05018 05024 05043 05055 60270 05070 05085 05095 05098 05103 40250 05110 05125 05145 05210 05250 05255 05300 05310 05320 05335 05347 05355 05370 05375 05390 05393 05394 05415 05395 05405 05445 05465 60285 05533 BRICANYL BROMANATE BROMANYL EXPECTORANT BROMATAPP BROMFED BROMOCRIPTINE BROMPHENIRAMINE BROMPHENIRAMINE COMPOUND ELIXIR BRONCHODILATOR BRONDECON BRONKAID BRONKOLIXIR BRONKOMETER BRONKOSOL BRONKOTABS BUCLADIN-S SOFTAB BUF ACNE CLEANSING BAR BUFFERIN BUFFERIN ARTHRITIS STRENGTH BUFFERIN W/CODEINE BUMEX BURN OINTMENT BUROW'S SOLUTION BUSPAR BUTABARBITAL BUTAL BUTALBITAL BUTALBITAL W/AC BUTALBITAL W/CODEINE BUTALBITAL/ASPIRIN/CAFFEINE BUTAZOLIDIN BUTIBEL BUTISOL C-RON FORTE CAFERGOT CAFERGOT P-B CAL-NOR CALADRYL CALAMINE CALAMINE LOTION CALAN CALCET CALCIDRINE CALCIFEROL CALCIMAR CALCITONIN CALCITREL CALCIUM CALCIUM ACETATE CALCIUM CARBONATE CALCIUM LACTATE CALCIUM-D CALEL-D CALORIC AGENT 40285 05543 05550 05570 05598 05600 05640 05645 05648 60290 41875 05663 05668 05675 05680 05690 40290 05750 05758 05760 05785 05788 05789 05810 05820 05870 05885 05895 05900 05903 05908 05955 05975 05983 05990 06005 06030 06050 06095 06100 06110 06120 06125 06128 06130 06133 06135 06170 06190 06210 06215 06225 06260 06265 CALTRATE CALTRO CAMA CAMPHOR CANTHARONE CANTIL CAPITAL W/CODEINE CAPITROL CAPOTEN CAPOZIDE CAPTOPRIL CARAFATE CARAMIPHEN W/PP CARBACHOL CARBAMAZEPINE CARBENICILLIN CARBODEC CARDEC-DM CARDIAC AGENT CARDILATE CARDIOQUIN CARDIOVASCULAR AGENT CARDIZEM CARISOPRODOL CARMOL CASCARA SAGRADA CASTOR OIL CATAPRES CATARASE CATHARTIC AGENT CDP PLUS CECLOR CEENU CEFADROXIL CEFOL CELESTONE CELONTIN CENAFED CENTRAX CENTRUM CEPACOL CEPASTAT CEPHALEXIN CEPHALOSPORINS CEPHALOTHIN CEPHRADINE CEPHULAC CERUMENEX CETAMIDE CETAPHIL CETAPRED CETYL ALCOHOL CHARCOAL CHARCOCAPS 06275 06294 40335 06300 06374 40350 06400 06405 06415 06430 06440 06455 06465 06470 06495 06500 06503 06520 06535 06540 06555 06565 06570 06580 06590 06595 06605 60310 06620 06625 06645 06663 06665 06680 06695 06710 06720 06734 06745 06755 06780 06800 06815 06838 06839 06860 06865 60325 06905 06913 06920 06930 40410 06935 CHARCOTABS CHEMOTHERAPY CHEMSTRIP UGK CHERACOL CHILDREN'S TYLENOL CHLOR-PRO CHLOR-TRIMETON CHLOR-TRIMETON DECONGESTANT CHLOR-TRIMETON REPETAB CHLORAFED CHLORAL HYDRATE CHLORAMBUCIL CHLORAMPHENICOL CHLORASEPTIC CHLORDIAZEPOXIDE CHLORDIAZEPOXIDE HCL W/CLIDINIUM BROM CHLORDINIUM SALTS CHLOROFON-F CHLOROMYCETIN CHLOROMYCETIN OTIC CHLOROMYCETIN-HYDROCORTISONE CHLOROPHYLL CHLOROPTIC CHLOROQUINE CHLOROTHIAZIDE CHLOROTHIAZIDE W/RESERPINE CHLORPHENIRAMINE CHLORPHENIRAMINE W/PHENYLPROPANOLAMIN CHLORPRAMAZINE CHLORPROPAMIDE CHLORTHALIDONE CHLORZOXAZONE W/ACETAMINOPHEN CHLORZOXAZONE W/APAP CHOLAN HMB CHOLEDYL CHOLESTYRAMINE RESIN DRIED CHOLINE CHOLINERGIC BLOCKING AGENT CHOLOXIN CHOREX CHROMAGEN CHRONULAC SYRUP CIMETIDINE CINOBAC PULVULE CIPRO CITRATE OF MAGNESIA CITRIC ACID CITRUCEL CLEOCIN CLEOCIN T CLINDAMYCIN CLINDEX CLINITEST CLINORIL 06940 06945 06968 06970 06975 06980 06985 06990 06993 07003 07010 60335 07015 60340 07067 07075 07180 07185 07190 07195 07200 07215 07250 07265 07270 07275 07280 07285 07293 07300 07313 07315 07330 07355 07405 07410 60355 07435 07465 07467 07470 07478 07480 07495 07528 07535 07543 07553 07560 04368 60370 07660 07665 07670 CLIPOXIDE CLISTIN CLODERM CLOFIBRATE CLOMID CLONAZEPAM CLONIDINE CLONOPIN CLORAZEPATE CLOTRIMAZOLE CLOXACILLIN CLOXACILLIN CLOXAPEN CO-GESIC CO-TRIMOXAZOLE COAL TAR CODEINE CODEINE PHOSPHATE CODEINE SULFATE CODIMAL CODIMAL DH SYRUP CODIMAL L.A. COGENTIN COLACE COLBENEMID COLCHICINE COLCHICUM COLD CAPSULE COLD RELIEF COLD TABLET COLDRINE COLESTID COLLYRIUM COLREX COLY-MYCIN COLY-MYCIN S COLYTE COMBIPRES COMHIST COMPAL COMPAZINE COMPOUND W COMTREX CONAR CONGESS CONHIST CONJUGATED ESTROGENS CONSTANT-T CONTAC CONTRACEPTIVE AGENT CORDARONE CORDRAN CORDRAN-N CORGARD 07680 07740 07745 07755 07760 07770 07775 07778 07780 07795 07800 60385 07805 07820 40445 07888 07910 07913 07920 07930 07945 07960 07990 08015 08040 08060 08065 08090 08120 08130 08135 08140 08145 08150 40455 08160 08175 08180 08205 08230 08245 08280 08285 08345 08350 08355 08369 08385 08390 08397 08410 08420 08425 08440 CORICIDIN CORRECTOL CORT-DOME CORTAID CORTEF CORTENEMA CORTICAINE CORTICOTROPIN CORTIFOAM CORTISONE CORTISPORIN CORTISPORIN OPHTHALMIC CORTONE CORTROSYN CORZIDE COTRIM COTYLENOL COUGH FORMULA COUGH SYRUP COUMADIN COVANGESIC CPA CREAMALIN CROMOLYN CRYSTODIGIN CUPRIMINE CURRETAB CYANOCOBALAMIN CYCLANDELATE CYCLAPEN CYCLOCORT CYCLOGYL CYCLOMYDRIL CYCLOPAR CYCLOPENTOLATE CYCLOSPASMOL CYLERT CYPROHEPTADINE CYSTOSPAZ CYTOMEL CYTOXAN D.S.S. D.S.S. W/CASANTHRANOL DACRIOSE DAILY MULTIPLE VITAMIN DAILY MULTIPLE VITAMIN W/IRON DALCAINE DALLERGY DALMANE DAMASON-P DANOCRINE DANTRIUM DANTROLENE DAPSONE 08445 08455 08460 08470 08475 08480 08490 08510 08535 08545 08565 08585 08590 08608 08605 08625 08635 08640 08660 08670 08680 08695 08700 08705 08730 08745 08753 08765 08770 08775 08780 08785 08795 08805 08810 08835 08836 08840 08860 08865 08870 08875 08880 08885 60400 40515 08905 08910 08915 08935 08918 08955 08960 08975 DARANIDE DARBID DARICON DARVOCET-N DARVON DARVON COMPOUND DARVON-N DATRIL DDAVP DE CAL DEBROX DECADRON DECADRON ELIXIR DECADRON TURBINAIRE DECADRON-LA DECASPRAY DECHOLIN DECLOMYCIN DECONAMINE DECONGESTANT DECONGESTANT ELIXIR DECONGEX DEGEST 2 DEHIST DELATESTRYL DELESTROGEN DELSYM DELTALIN DELTASONE DEMAZIN DEMEROL DEMEROL SYRUP DEMI-REGROTON DEMULEN DEMULEN-28 DEPAKENE DEPAKOTE DEPEN DEPO-ESTRADIOL DEPO-MEDROL DEPO-PROVERA DEPO-TESTADIOL DEPO-TESTOSTERONE DEPOGEN DEPONIT DEPROIST W/CODEINE DEPROL DERFULE DERIFIL DERMA PH LOTION DERMACAINE DERMACORT DERMAL RUB DERMAVAL 09000 09010 09015 09020 09040 09045 09050 40520 09058 09060 60405 40525 09075 09080 09085 09088 60415 09110 09116 09117 35545 35550 09118 09120 09125 09170 09173 09175 09185 09190 09220 42245 40580 09250 09273 09290 09295 09305 09343 09352 09360 09365 09370 09385 09390 09395 09425 09433 09435 09445 09455 09460 09465 09470 DERMOPLAST DESENEX DESFERAL DESIPRAMINE DESOXYN DESQUAM-X DESQUAM-X WASH DESYREL DETUSS DETUSSIN DEXACIDIN DEXAIR DEXAMETHASONE DEXAMETHASONE ACETATE DEXAMETHASONE ELIXIR DEXAMETHASONE OPHTH DEXAMETHASONE/NEOMYCIN/POLYMIX DEXAPED DEXASPORIN OPHTHALMIC DEXBROMPHENI/PSEUDOEPHED DEXBROMPHENIRAMINE DEXBROMPHENIRAMINE/D-ISOEPHED DEXCHLORPHENIRAMINE DEXEDRINE DEXON DEXTROAMPHETAMINE DEXTROMETHORPHAN COUGH DEXTROSE DEXTROSTIX DEZONE DI-GEL DI-GESIC DIABETA DIABINESE DIAGARD DIALOSE DIALUME DIAMOX DIARAL DIASORB DIASTIX DIATRIZOATE DIAZEPAM DIBENZYLINE DIBUCAINE DICAL-D DICARBOSIL DICLOXACILLIN DICODID DICUMAROL DICYCLOMINE DICYCLOMINE HCL W/PHENOBARBITAL DIDREX DIDRONEL 09475 09490 09495 09500 09535 09540 09545 09550 09555 09585 09590 40590 09593 09600 09625 09630 09635 09638 09640 09650 09660 09670 09675 09680 09690 09697 09710 09720 09730 09735 09770 09775 09823 09835 09838 09850 09840 09860 09875 40605 40610 09880 09890 09990 40615 09915 09920 09925 09935 09945 09955 60470 09970 09975 DIENESTROL DIETHYL-PROPION DIETHYLPROPION DIETHYLSTILBESTROL DIGITALIS DIGITOXIN DIGOXIN DIHISTINE DIHISTINE ELIXIR DILANTIN DILANTIN W/PHENOBARBITAL DILATAIR DILATRATE DILAUDID DILOR DILOR-G DIMACOL DIMALIX DIMENHYDRINATE DIMENTABS DIMETANE DIMETANE EXPECTORANT DIMETANE EXPECTORANT-DC DIMETANE EXTENTAB DIMETAPP DIMOTAL DIOCIO DIOCIO SYRUP DIOCTYL SODIUM SULFOSUCC W/CASANTHRAN DIOCTYL SODIUM SULFOSUCCINATE DIONIN DIOSTATE D DIPHENADRIL DIPHENATOL DIPHENCEN DIPHENHYDRAMINE DIPHENHYDRAMINE COMPOUND EXPECTORANT DIPHENHYDRAMINE HCL ELIXIR DIPHENHYDRAMINE HCL 22 GA DIPHENOXY/ATROP DIPHENOXYLATE DIPHENOXYLATE HCL & ATROPINE SULFATE DIPHENYLHYDANTOIN SODIUM DIPHTHERIA TETANUS TOXOIDS PERTUSSIS DIPROLENE DIPROSONE DIPYRIDAMOLE DISALCID DISIPAL DISOLAN DISOPHROL DISOPYRAMIDE PHOSPHATE DISPATABS DISULFIRAM 09980 09995 10000 10005 10010 10015 10020 10025 10030 10040 10045 10087 10089 40625 10115 10126 10130 10140 10145 10150 10180 10200 10205 10210 10213 10215 10220 10230 10250 10255 60485 10273 10275 10285 10325 10330 10335 10340 10364 60500 10355 10358 10380 10440 10455 10485 10490 10518 10575 10605 10615 10620 10630 10650 DITAN DITROPAN DIUCARDIN DIULO DIUMEAD DIUPRES DIURETIC DIURIL DIUTENSEN DM-PLUS COUGH SYRUP DOAK OIL DOCUSATE DOCUSATE CALCIUM DOCUSATE POTASSIUM DOLENE DOLOBID DOLOPHINE DOME-PASTE BANDAGE DOMEBORO DOMEBORO OTIC DONATUSSIN DONNAGEL DONNAGEL-PG DONNATAL DONNATAL NO.2 DONNAZYME DONPHEN DOPAR DORC0L PEDIATRIC COUGH SYRUP DORIDEN DORYX DOSS COMPOUND PLUS DOSS 300 DOVACET DOXEPIN DOXIDAN DOXINATE DOXY C DOXY-TABS DOXY-100 DOXYCYCLINE DOXYLAMINE B-6 DRAMAMINE DRISDOL DRISTAN DRIXORAL DRIZE DRYSOL DULCOLAX DUO-C.V.P. DUO-K DUO-MEDIHALER DUOFILM DUOLUBE 10675 10705 10755 10758 10760 10765 10800 10803 10805 10810 10815 10820 10835 10840 10845 10865 10875 10895 10905 10910 10925 10948 10949 10970 10975 10985 11005 11025 11045 11050 11065 11075 11095 11100 11120 11130 11140 11145 11150 11152 11190 11210 11230 11245 11250 11260 11265 11268 11325 11340 11345 11350 11372 11390 DUOVENT DURACILLIN DURAQUIN DURASIL DURATEARS DURATEST DURICEF DURRAX DUVOID DV DYAZIDE DYCILL DYMELOR DYMENATE DYNAPEN DYRENIUM E.E.S E-FEROL E-MYCIN E-PILO EAR DROPS EASPRIN EAZOL ECONOPRED ECOTRIN EDECRIN EFFERSYLLIUM EFUDEX ELASE ELASE-CHLOROMYCETIN ELAVIL ELDEC ELDERCAPS ELDERTONIC ELIXIR ELDOQUIN ELECTROLYTE ELIXOPHYLLIN ELIXOPHYLLIN S.R. ELIXOPHYLLIN-KI ELOCON EMETROL EMPIRIN EMPIRIN COMPOUND #3 EMPIRIN NO. #2 EMPIRIN NO. #3 EMPIRIN W/CODEINE EMPRACET EMPRACET W/CODEINE ENDEP ENDURON ENDURONYL ENDURONYL FORTE ENKAID ENSURE 11395 11400 11433 11450 11525 11530 11540 11548 11555 11580 11585 11616 11635 11640 11645 11658 11651 60535 11653 40725 11655 11657 11668 11660 11665 11688 11690 11695 11700 11725 11740 60545 11745 11760 11765 11770 11800 11815 11845 11850 11855 11860 11865 11870 11920 11945 11960 11965 11975 11980 12035 11978 12050 12055 ENTEX ENTOZYME ENZYME EPHEDRINE EPIFRIN EPINAL EPINEPHRINE EPIPEN EPPY EQUAGESIC EQUANIL ERGOLOID MESYLATES ERGOSTAT ERGOTAMINE ERGOTRATE ERY-TAB ERYC ERYCETTE ERYDERM ERYMAX ERYPAR ERYPED ERYTHRO-RX ERYTHROCIN ERYTHROMYCIN ESGIC ESIDRIX ESIMIL ESKALITH ESTINYL ESTRACE ESTRADERM ESTRADIOL ESTRATAB ESTRATEST ESTRAVAL ESTROGEN ESTRONE ESTROVIS ETHAMBUTOL ETHAMIDE ETHAQUIN ETHATAB ETHAVERINE HCL ETRAFON EUCERIN EURAX EUTHROID EUTRON EVAC-Q-KIT EXCEDRIN EXPECTORANT EXSEL LOTION EXTENDRYL 60550 12078 50025 12134 12140 12150 12160 12193 12225 60560 12235 12255 12260 12275 12285 12290 12300 12305 12310 12340 12345 12350 12410 60575 12415 12430 12465 12480 12490 60590 12510 12515 12523 12525 12530 12535 12540 12545 60595 12550 12565 12570 12585 12620 12635 12663 12665 12670 12690 12695 12730 12735 12740 12750 EYE DROPS EYE PREPARATION FAMILY TABS WITH IRON FANSIDAR FASTIN FEBRINOL FEDAHIST FELDENE FEMIRON FEMSTAT FENDOL FEOSOL FEOSOL ELIXIR FEOSTAT FER-IN-SOL FERANCEE FERGON FERGON ELIXIR FERGON PLUS FERO-FOLIC-500 FERO-GRAD-500 FERO-GRADUMET FERRO FERRO BC FERRO-SEQUEL FERROLIP FERROUS FUMARATE FERROUS GLUCONATE FERROUS SULATE FERROUS-DS FESTAL FESTALAN FIBERMED FILIBON FILIBON F.A. FILIBON FORTE FILIBON OT FIOGESIC FIORICET FIORINAL FIORINAL NO. 3 FIORINAL W/CODEINE FLAGYL FLEET ENEMA FLEXERIL FLORICAL FLORINEF FLORONE FLUOCINOLONE FLUOGEN FLUORI-METHANE SPRAY FLUORIDE FLUORIDENT FLUORINSE 12755 12763 12765 12780 12785 12805 12810 12825 12845 42380 40755 12865 12880 12905 12920 12940 12945 12955 12963 40760 12970 12975 12995 13045 13085 13096 13100 13105 13118 13120 13153 13170 13195 13200 13205 13208 13209 13220 13245 13295 13300 13310 40775 60635 13320 60640 13325 13350 13390 13440 13455 13465 13495 13530 FLUORITAB FLUOROMETHOLONE FLUOROPLEX FLUPHENAZINE FLURA-DROPS FLURA-VITE FLURAZEPAM FLUROSYN FML LIQUIFILM FML-FORTE OPHTHALMIC FOLEX FOLIC ACID FOLVITE FORCOLD SYRUP FORMALIN FORMULA 2 FORMULA 44 COUGH MIXTURE FORMULA 44-D COUGH MIXTURE FORTA FORTABS FORTESPAN FOSFREE FOSTRIL FULVICIN FUNGIZONE FUNGOID FURACIN FURADANTIN FUROSEMIDE FUROXONE GALLBLADDER MEDICATION GAMMAR GANTANOL GANTRISIN GARAMYCIN GARGLE GASTRIC AGENT GAVISCON GEL-KAM GELUSIL GEMONIL GENOPTIC GENTACIDIN GENTAFAIR GENTAMICIN GENTAMICIN OPHTHALMIC GENTIAN VIOLET GEOCILLIN GERIATRIC ELIXIR GERIPLEX GERITOL GERIX ELIXIR GEVRABON GLAUCON 13550 13553 13585 13595 60665 41820 13640 13675 13705 13715 13750 13752 42425 13775 13780 13785 13790 13795 13800 13825 13818 13835 13830 13838 13845 13850 13865 13875 13880 13885 13890 13895 13930 40785 13955 13980 13995 13999 14000 14005 14035 60675 14040 14045 14050 14135 14180 14190 14195 14203 14240 14275 14279 14320 GLUCOSE GLUCOTROL GLUTOL GLY-OXIDE GLYCERIN GLYCERIN SUPPOSITORIES GLYCINE GLYCOTUSS GLYDEINE SYRUP GLYESTRIN GOLD SODIUM THIOSULFATE GOLYTELY GOLYTELY GRANULEX GREEN SOAP GRIFULVIN GRIS-PEG GRISACTIN GRISEOFULVIN GUAIATRATE SYRUP GUAIFED GUAIFENESIN GUAIFENESIN & DEXTROMETHORPHAN HBR GUAIFENESIN W/CODEINE GUANETHIDINE GUANIDINE GUIATEX GUIATUSS GUIATUSS A.C. SYRUP GUIATUSSIN GUIATUSSIN W/CODEINE GUIATUSSIN W/DEXTROMETHORPHAN GYNE-LOTRIMIN H.E.A. H.H.R. H-H HALAZONE HALCION HALDOL HALDRONE HALOG HALOG-E HALOPERIDOL HALOTESTIN HALOTEX HEMATINIC HEMORRHOIDAL HEMORRHOIDAL HC HEMORRHOIDAL SUPPOSITORY HEMOSTATIC AGENT HEPARIN HEPRON HEPTAVAX-B HEXACHLOROPHENE 14340 14370 14378 14425 14440 14489 14500 14505 14510 14515 14535 14545 14550 14595 14625 14645 14683 14713 14717 14720 14725 14727 41895 14770 14768 14790 14795 14805 60710 14820 14840 14855 14860 14870 14875 14895 14900 14888 14905 14917 40845 14935 14945 14955 14960 14903 14965 14980 14985 40850 14990 15002 15010 15020 HEXADROL HEXAVITAMIN HHR HIGH POTENCY B-VITAMINS W/C NEO-VADRI HIPREX HISTAFED C HISTALET HISTALET FORTE HISTALET SYRUP HISTALET X HISTAMINE HISTASPAN HISTASPAN-D HISTOPLASMIN DILUTED SOLUTION HMS HOMATROPINE HORMONE HUMAN CHORIONIC GONADOTROPIN HUMATROPE HUMIST SPRAY HUMORSOL HUMULIN HUMULIN INSULIN HYCODAN HYCODAPHEN HYCOFF HYCOMINE HYCOTUSS HYDEGINE LIQUID CAPSULE HYDERGINE HYDRALAZINE HYDRALAZINE-HYDROCHLOROTHIAZIDE-RESER HYDRAMINE HYDRATE HYDREA HYDRO-CHLOR HYDRO-CHLORO-THIAZIDE HYDRO-ERGOT HYDRO-RESERPINE HYDROCET HYDROCHLOR/RESERPINE/HYDRAL HYDROCHLOROTHIAZIDE W/RESERPINE HYDROCIL HYDROCOCONE HYDROCODONE SYRUP HYDROCORT HYDROCORTISONE HYDROCORTONE HYDRODIURIL HYDROFLUMETHAZIDE HYDROGEN PEROXIDE HYDROMINE HYDROMOX HYDROPHED 15025 15035 15043 15045 15058 15070 15100 15095 15105 15115 15138 41840 15160 15238 15240 15243 15285 15305 15307 15320 15370 15375 15380 15395 15455 15460 15475 60730 15490 15495 15515 15520 15545 15555 15570 15575 15580 15590 15600 15615 15630 15678 15680 15685 15710 15727 15728 15730 15755 15760 15773 15780 60750 60755 HYDROPHILIC OINTMENT HYDROPRES HYDROSERP HYDROSERPINE HYDROXACEN HYDROXYCHLOROQUINE HYDROXYZINE HYDROXYXINE COMPOUND SYRUP HYDROXYZINE PAMOATE HYGROTON HYLOREL HYOSCINE HYOSOPHEN HYPNOTIC AGENT HYPOTEARS HYPOTENSIVE AGENT HYTAKEROL HYTONE HYTRIN HYZINE IBERET IBERET-FOLIC-500 IBERET-500 IBUPROFEN ICY HOT ANALGESIC BALM IDENAL ILETIN ILETIN II ILOSONE ILOTYCIN IMFERON IMIPRAMINE IMODIUM IMURAN INCREMIN W/IRON INDERAL INDERIDE INDOCIN INDOMETHACIN INFLAMASE INH INSULATARD NPH INSULIN INTAL INULIN IODINATED GLYCEROL W/CODEINE IODINATED GLYCEROL W/DM IODINE IODOCHLOR W/HYDROCORTISONE IODOCHLORHYDROXYQUIN IODOFORM IONAMIN IOPHEN IOPHEN DM 15798 15865 15870 15885 15920 15930 15945 15955 15960 15965 15980 15985 15990 16003 16025 40905 16035 16040 16045 16050 16055 16070 16075 16095 16105 16115 16130 16140 60775 16210 41915 16215 16220 16222 16245 16268 16310 16315 16325 16340 16360 16365 16380 16385 16390 16415 16455 41850 16472 16475 16480 16482 16485 16490 IOPHEN-C IRON & B COMPLEX PLUS IRON PREPARATION IRON W/VITAMIN C ISMELIN ISO-BID ISOCLOR ISOCLOR TIMESULE ISODINE ISOETHARINE ISOLLYL ISOLYTE ISONIAZID ISOPHANE INSULIN SUSPENSION ISOPROTERENOL ISOPTIN ISOPTO ATROPINE ISOPTO CARBACHOL ISOPTO CARPINE ISOPTO CETAMIDE ISOPTO CETAPRED ISOPTO HOMATROPINE ISOPTO HYOSCINE ISORDIL ISOSORBIDE ISOXSUPRINE ISUPREL ISUPREL HCL MISTOMETER K-DUR K-LOR K-LYTE K-LYTE DS K-LYTE/CL K-NORM K-PHOS K-TAB KANTREX KANULASE KAOCHLOR KAOLIN & PECTIN KAON KAOPECTATE KAPECTOLIN KAPECTOLIN PG KAPECTOLIN W/BELLADONNA KARIDIUM KAYEXALATE KCL KEFLET KEFLEX KEFLIN KEFTAB KEFZOL KELEX 16495 16500 16505 16520 16525 16595 16635 16640 60790 16710 16720 16725 16728 16750 16765 16780 16785 16800 50030 16845 16865 16870 16895 40908 41890 16905 60795 16915 16923 16940 16950 16955 60805 16975 17055 17070 17113 17115 17130 17145 17150 17160 17165 17243 17265 17270 17275 17304 17325 17322 60815 17340 17365 17370 KEMADRIN KENACORT KENALOG KERALYT KERI KETO-DIASTIX KIE KINESED KLONOPIN KLOR-CON KLORVESS KLORVESS EFFERVESCENT GRANULE KLOTRIX KOLYUM KOMED KONAKION KONDREMUL KONSYL KRONOFED-A KU-ZYME KUTRASE KWELL L-THYROXINE L-TRYPTOPHANE L/DOPA LABID LACHYDRIN LACRI-LUBE LACRISERT OPHTH LACTIC ACID LACTINEX LACTOBACILLUS ACIDOPHILUS LACTRASE LACTULOSE LANIAZID LANOLIN LANOXICAPS LANOXIN LANVISONE LARODOPA LAROTID LASAN LASIX LAXATIVE LECITHIN LEDERCILLIN VK LEDERPLEX LENTE INSULIN SUSPENSION LEUKERAN LEUKOVORIN CALCIUM LEVLEN LEVO-DROMORAN LEVOTHROID LEVOTHYROXINE 17375 17380 17390 17395 17410 17440 17445 17450 17475 17485 17530 17540 17548 17560 17595 17600 17630 17668 17670 17680 17715 17725 17730 17735 17765 17820 17825 17834 17840 17860 17865 17870 17875 17878 17880 17883 17885 40950 17889 17888 17890 17925 60835 17930 17945 40955 17975 17978 17980 17990 18000 18010 60845 18020 LEVSIN LEVSIN-PB DROPS LEVSINEX LEVSINEX/PHENOBARBITAL LEXOR LIBRAX LIBRITABS LIBRIUM LIDEX LIDOCAINE LIMBITROL LINCOCIN LINDANE LIORESAL LIPODERM LIPOFLAVONOID LIPOTRIAD LIQUID PRED LIQUIFILM LIQUIPRIN LITHIUM LITHOBID LITHONATE LITHOTABS LIVITAMIN LO-TUSSIN SYRUP LO/OVRAL LOCOID LOESTRIN LOMANATE LOMOTIL LONALAC LONITEN LONOX LOPERAMIDE LOPID LOPRESSOR LOPROX LOPURIN LORAZEPAM LORELCO LOTRIMIN LOTRISONE LOTUSATE LOXITANE LOZOL LUBRIDERM LUDIOMIL LUFA LUFYLLIN LUFYLLIN-GG LUGOL'S SOLUTION LURIDE LURIDE DROPS 18050 18070 18125 18130 18159 18195 18200 18215 18221 18315 18365 18375 18440 18445 18480 18490 18515 18518 35555 18520 60875 18523 18781 18550 18552 18553 18555 18556 18558 18595 60880 18610 18620 60885 60890 18635 18640 18643 18655 40975 18664 18665 18670 18685 18695 18698 18735 18745 18755 18760 18775 18795 18798 18800 LYSINE LYTREN MAALOX MACRODANTIN MAGNACAL MAGNESIUM CITRATE MAGNESIUM GLUCONATE MAGNESIUM SULFATE MAGNESIUM/ALUMINUM HYDROXIDE MANDELAMINE MANTADIL MARAX MARNAL MARPLAN MATERNA MATULANE MAXIDEX MAXIFLOR MAXIMUM STRENGTH ASPIRIN MAXITROL MAXIVATE MAXZIDE ME-PREDNISOLONE MEBARAL MEBENDAZOLE MECLAN MECLIZINE MECLOFENAMATE SODIUM MECLOMEN MEDIATRIC MEDICATED PADS MEDICONE DRESSING MEDIHALER-EPI MEDIPLAST PLASTERS MEDIPREN MEDRALONE MEDROL MEDROXYPROGESTERONE MEGACE MEGATON MELANEX MELFIAT MELLARIL MELPHALAN MENEST MENI-D MENRIUM MENTHOL MEPERGAN MEPERIDINE MEPHYTON MEPROBAMATE MEPROGESIC MEPROSPAN 18820 60895 18900 18910 18915 18920 18925 18930 18935 18940 18945 18960 18965 18985 19035 19045 19070 19090 19100 19118 19140 19155 60905 19175 19178 19180 19185 19200 19205 19208 19210 19218 19230 19231 60915 19233 19234 19242 60920 40990 19270 19280 19290 19305 19313 50035 19320 19337 19343 19360 19375 19405 19415 19445 MERCAPTOPURINE MERITAL MERTHIOLATE MERVALDIN MESANTOIN MESTINON METAHYDRIN METAMUCIL METANDREN METAPREL METAPROTERENOL METAJENSIN METH METHADONE METHENAMINE METHERGINE METHOCARBAMOL METHOTREXATE METHOXANOL METHYCLOTHIAZIDE METHYLCELLULOSE METHYLDOPA METHYLDOPA W/HCTZ METHYLPHENIDATE METHYLPRED-40 METHYLPREDNISOLONE METHYLTESTOSTERONE METICORTEN METIMYD METOCLOPRAMIDE METOLAZONE METOPROLOL METRETON METRO METRODIN METRONIDAZOLE METRYL MEVACOR MEXITIL MG-AL HYDROX W/SIMETH MI-CEBRIN MICATIN MICONAZOLE MICRIN PLUS MICRO-K EXTENCAPS MICRONASE MICRONOR MICROX MIDAMOR MIDRIN MILK OF MAGNESIA MILPATH MILTOWN MINERAL OIL 19455 42705 60925 60930 19460 19465 19478 19490 19553 19568 19595 19600 19605 19610 19618 19625 19633 19635 42720 19644 19648 19650 19675 19698 19710 19715 19735 19780 19795 19835 19853 19925 60955 41135 19948 19995 20000 20010 20035 20045 20055 20060 20065 20070 20075 20080 20095 20105 20110 20135 20140 20153 60985 41145 MINIPRESS MINIZIDE MINIZIDE 1 MINIZIDE 2 MINOCIN MINOCYCLINE MINOXIDIL MINTEZOL MITROLAN MIXTARD MODANE MODERIL MODICON MODICUM MODURETIC MOL-IRON MOMENTUM MONISTAT MONISTAT-DERM MONO-GESIC MONOTARD MORPHINE MOTRIN MOXAM MUCILOSE MUCOMYST MUDRANE MULTI-VI-FLOR DROPS MULTICEBRIN MULTIPLE VITAMINS W/MINERALS MULTIVITAMIN/FLUORIDE MURINE MURO TEARS MURO'S OPCON-A MUSCLE RELAXANT MYADEC MYAMBUTOL MYCELEX MYCITRACIN MYCO TRIACET MYCOLOG MYCOSTATIN MYDFRIN MYDRAPRED MYDRIACYL MYLANTA MYLICON MYOCHRYSINE MYOFLEX MYSOLINE MYSTECLIN F MYTREX NA SAL NAFAZAIR 20190 20195 20200 20210 20215 20220 20255 20260 20270 20285 20290 20300 20315 20320 20325 41150 20338 20360 20365 20370 20385 20390 20395 20415 20420 20435 20440 20490 20505 20530 20555 20580 20585 20595 20655 61015 20688 20690 20730 42780 20760 20770 20795 20798 20805 20818 20835 20855 20885 20890 20935 20945 20950 20960 NALDECON NALDEGESIC NALDELATE SYRUP NALFON NALIDIXIC ACID NAMIDE NAPHAZOLINE NAPHCON NAPHCON-A NAPROSYN NAPROXEN NAQUA NARDIL NASAHIST NASAL DECONGESTANT NASALCROM NASALIDE NATABEC NATABEC RX NATABEC W/FLUORIDE NATAFORT NATALINS NATALINS RX NATURETIN NATURETIN W/K NAVANE NAZAC DECONGESTANT NEGGRAM NEMBUTAL NEO-CORTEF NEO-MEDROL NEO-POLYCIN NEO-SYNALAR NEO-SYNEPHRINE NEODECADRON NEODEXAIR NEOM/POLY M GRAMICIDIN OPHTH NEOMYCIN NEOSPORIN NEOSPORIN OPHTHALMIC NEOTHYLLINE NEOTRIZINE NEPHRAMINE NEPHROCAPS NEPTAZANE NETROMYCIN NEUTRA-PHOS NEUTROGENA NIACIN NIACINAMIDE NICO-SPAN NICO-400 NICOBID NICOLAR 61025 20970 20980 21010 21065 21125 21130 21143 21144 21145 61030 21155 21160 21165 21170 21175 21185 61035 21203 21205 21213 21215 21220 21225 21228 21280 21290 21300 21330 21335 21340 42800 21373 61040 21385 21390 21400 21403 61620 21405 21425 21430 21440 21445 21450 21455 21460 21465 21500 61045 41855 21530 21535 41205 NICORETTE NICOTINAMIDE NICOTINIC ACID NIFEREX NILSTAT NITRO T.D. NITRO-BID NITRO-DUR NITRODISC NITROFURANTOIN NITROGARD NITROGEN NITROGLYCERIN NITROGLYN NITROL NITROLIN NITROSTAT NIX NIZORAL NOCTEC NOLAHIST NOLAMINE NOLUDAR NOLVADEX NORDETTE NORFLEX NORGESIC NORINYL NORLESTRIN NORLUTATE NORLUTIN NORMAL SALINE NORMODYNE NOROXIN NOROXINE NORPACE NORPRAMIN NORTRIPTYLINE NORWICH ASPIRIN NOSE DROPS NOVAFED NOVAFED A NOVAHISTINE NOVAHISTINE COUGH FORMULA NOVAHISTINE DH NOVAHISTINE DMX NOVAHISTINE ELIXIR NOVAHISTINE EXPECTORANT NOVOCAIN NOVOLIN NPH INSULIN NU-IRON NU-IRON ELIXIR NU-TEARS 21550 21555 21585 21590 61060 21605 21650 21670 21695 21700 21701 21698 21703 21750 21752 42835 61070 21754 41210 61075 21755 21765 21795 21802 21850 21855 21893 21900 21920 21930 21970 21995 22005 22015 22030 22055 22060 22065 22080 22090 22095 61100 22110 22120 22125 22170 41220 22180 22185 22195 22200 22210 22215 22220 NUBAIN NUCOFED NUPERCAINAL NUPERCAINE NUPRIN NUTRACORT NYLIDRIN NYQUIL NYSTATIN NYSTATIN VAGINAL TABLET NYSTATIN W/TRIAMCINOLONE NYSTATIN/NEO/GRAMICD/TRIAMCIN NYSTEX OCEAN MIST OCTAMIDE OCTICAIR OCTICAIR SOLUTION OCUFEN OCUGESTRIN OCUMYCIN OCUSOL OGEN OMNIPEN ONE-A-DAY-ESSENTIAL OPHTHOCHLOR OPHTHOCORT OPTICROM OPTIMINE ORABASE HCA PASTE ORABASE W/BENZOCAINE PASTE ORASONE ORETIC ORETON ORGANIDIN ORINASE ORNADE ORNEX ORPHENADRINE ORTHO-CREME ORTHO-NOVUM ORTHOXICOL ORUDIS OS-CAL OS-CAL 500 OS-CAL-FORTE OTIC-HC OTICAIR OTOBIOTIC OTOCORT OTOREID-HC OTRIVIN OVCON OVRAL OVRETTE 22225 22233 22242 22270 22273 22303 22305 22306 22308 22315 22323 22338 22340 22348 22350 61125 22410 22435 22470 61130 22490 22520 22578 22630 22635 22640 22670 22675 22690 22695 22730 22770 22805 22810 22840 22845 22860 22875 22885 22890 22910 22935 22945 22965 22975 23006 23010 23015 23045 23047 23803 61135 23100 23115 OVULEN OXACILLIN OXAZEPAM OXSORALEN OXTRIPHYLLINE OXYCODONE HCL OXYCODONE HCL & ACETAMINOPHEN OXYCODONE W/APAP OXYCODONE W/ASPIRIN OXYGEN OXYMETAZOLINE HCL OXYSTAT OXYTETRACYCLINE OYSCO 500 OYSTER SHELL & VITAMIN D P-A-C P-A-C COMPOUND P-V TUSSIN PABALATE PAIN RELIEVER PAIN RELIEVER-E PAMELOR PANADOL PANCREASE PANCREATIC HORMONE PANCREATIN PANMYCIN PANOXYL PANTERIC PANTHODERM PANTOTHENOL (D) ALCOHOL PAPAVERINE PARAFLEX PARAFON FORTE PAREDRINE W/BORIC ACID PAREGORIC PAREPECTOLIN PARLODEL PARNATE PARSIDOL PATHIBAMATE PATHOCIL PAVABID PAVAGEN PAVARINE PAXIPAM PAZO HEMORRHOID PBZ PBZ-SR PCE PEDI-CORT V PEDIACARE PEDIACOF PEDIAFLOR DROPS 23120 23125 61140 23140 23150 23158 23170 23185 23200 23210 23215 23220 23225 23228 23230 61145 23250 23285 23305 23315 61160 23370 23385 23390 23400 23415 23425 23430 23435 23440 23450 23490 23523 23524 23525 23535 23550 23560 23570 23610 23630 23645 23660 23670 23675 23680 23695 23700 23710 23715 23755 23760 23765 23770 PEDIALYTE PEDIAMYCIN PEDIAPRED PEDIATROL PEDIAZOLE PEDICULICIDE PEGANONE PEN-VEE, K PENAPAR VK PENICILLAMINE PENICILLIN PENICILLIN G PENICILLIN V PENICILLIN V POTASSIUM PENICILLIN VK PENNTUSS PENTAERYTHRITOL PENTAZOCINE PENTIDS PENTOTHAL PEPCID PEPTO-BISMOL PERCOCET-5 PERCODAN PERCOGESIC PERDIEM GRANULE PERGONAL PERI-COLACE PERI-DOSS PERIACTIN PERIDIN-C PERITRATE PERPHENAZINE PERPHENAZINE W/AMITRIPTYLINE PERSA-GEL PERSANTINE PERTOFRANE PERTUSSIN PETRO-PHYLIC SOAP PHAZYME PHEDRAL PHEN-DIMETRAZINE PHENAPHEN PHENAPHEN NO. 3 PHENAPHEN NO. 4 PHENAPHEN W/CODEINE PHENATAPP ELIXIR PHENATE PHENAZODINE PHENAZOPYRIDINE PHENERGAN PHENERGAN COMPOUND PHENERGAN EXPECTORANT PLAIN PHENERGAN EXPECTORANT W/CODEINE 23775 23780 23785 23790 23795 23823 23825 23845 23855 23870 23970 24005 24015 24018 24025 41280 24033 24045 24075 24080 24100 24120 24125 24130 61180 24140 24190 24195 41295 24280 24285 24300 24340 24395 24400 24410 24478 24415 24418 24420 24435 24465 24470 24510 24515 24525 24545 24550 24590 24595 24650 24695 24710 24765 PHENERGAN EXPECTORANT W/DEXTROMETHORP PHENERGAN SYRUP PHENERGAN VC EXPECTORANT PLAIN PHENERGAN VC EXPECTORANT W/CODEINE PHENERGAN-D PHENHIST PHENHIST DH W/CODEINE PHENOBARBITAL PHENOBARBITAL & BELLADONNA PHENOBARBITAL ELIXIR PHENTERMINE PHENYLBUATZONE PHENYLEPHRINE PHENYLHISTINE DH PHENYLPROPANOLAMINE PHENYLPROPANOLAMINE W/CPM PHENYLPROPYLAMIN/GUAIFENESIN PHENYTOIN PHISODERM PHISOHEX PHOS-FLUR PHOSPHATE ENEMA PHOSPHO-SODA PHOSPHOLINE PHOSPHOLINE IODIDE PHRENILIN PILOCAR PILOCARPINE PILOKAIR PLACEBO PLACIDYL PLAQUENIL PLEGINE POISON IVY SPRAY POLARAMINE POLLEN ANTIGEN POLY-PRED POLY-VI-FLOR POLY-VI-FLOR W/IRON POLY-VI-SOL POLYCILLIN POLYMOX POLYMYXIN POLYSPORIN POLYTAR POLYVITAMIN PONDIMIN PONSTEL POT CHLOR POTABA POTASSIUM POTASSIUM GLUCONATE POTASSIUM IODIDE POVIDONE 61205 24770 24780 24785 24790 24795 24804 24805 24830 24850 24855 41345 24885 24890 24910 24925 24945 24950 24960 24965 24975 24980 24985 24988 24995 25015 25025 25033 25040 25055 25065 25100 25115 25140 25150 25155 25180 25185 25195 25200 25213 25220 25240 25250 25255 25305 25330 25335 25338 25358 25359 25365 25375 25380 POVIDON-IODINE POXY COMPOUND-65 PRAGMATAR PRAMET FA PRAMILET FA PRAMOSONE PRAZEPAM PRAZOSIN PRE-NATAL VITAMINS PRED FORTE PRED MILD PREDAIR PREDNISOLONE PREDNISONE PREFRIN PREGNYL PRELUDIN PREMARIN PREMARIN VAGINAL PREMARIN W/METHYLTESTOSTERONE PRENATAL FORMULA (VITAMINS) PRENATAL STUART PRENATAL W/FOLIC ACID PRENATAL W/FOLIC ACID & IRON PREPARATION H PRESALIN PRESUN PREVIDENT LIME PRIMATENE MIST PRIMIDONE PRINCIPEN PRO-BANTHINE PRO-PHEN PROBEN-C PROBENECID PROBENECID W/COLCHICINE PROCAINAMIDE PROCAINE PROCAMIDE PROCAN SR PROCARDIA PROCHLORPERAZINE PROCTOCORT PROCTOFOAM PROCTOFOAM-HC PROGESTERONE PROLIXIN PROLOID PROLOPRIM PROMETH PROMETH W/COD PROMETHAZINE PROMETHAZINE COMPOUND W/CODEINE PROMETHAZINE EXPECTORANT 25385 25390 25400 25405 25410 25420 25430 25455 61240 25463 25470 25475 25493 25495 25510 25505 25515 25530 25545 42985 61245 25550 25560 25570 25575 41380 25618 25635 25638 25640 25660 25691 41928 25695 25696 61260 41830 41845 35575 35576 25750 25775 25800 25805 25810 25815 25825 25875 25880 25890 25900 25930 25945 25965 PROMETHAZINE EXPECTORANT DM PEDIATRIC PROMETHAZINE EXPECTORANT W/CODEINE PROMETHAZINE HCL EXPECTORANT W/CODEINE PROMETHAZINE HCL SYRUP PROMETHAZINE HCL VC EXPECTORANT PROMETHAZINE HCL W/DEXTROMETHORPHAN PROMETHAZINE VC W/CODEINE PRONESTYL PROPACET PROPAGEST PROPANOLOL PROPANTHELINE PROPINE PROPION GEL PROPOXYPHENE PROPOXYPHENE COMPOUND 65 PROPOXYPHENE HCL COMPOUND PROPOXYPHENE HCL W/ACETAMINOPHEN PROPOXYPHENE W/APAP PROPRANOLOL PROPRANOLOL HCL PROPYLENE GLYCOL PROPYLTHIOURACIL PROSOBEE PROSTAPHLIN PROTAMINE ZINC INSULIN PROTOSTAT PROVAL PROVENTIL PROVERA PROXENE PSEUDO-CAR DM PSEUDOEPHED/CABINOXAMINE/DM PSEUDOEPHEDRINE PSEUDOEPHEDRINE W/TRIPROLIDINE PSORCON PSYLLIUM PTU PURIFIED BEEF INSULIN PURIFIED PORK INSULIN PURINETHOL PYOCIDIN PYRAZINAMIDE PYRIDIATE PYRIDIUM PYRIDIUM PLUS PYRIDOXINE PYRROXATE PYRROXATE Q/CODEINE PHOSPHATE P2E1 P4E1 QUADRINAL QUARZAN QUESTRAN 41385 25970 25975 25980 26015 26020 41390 26025 26030 26035 41395 26040 26045 26055 26073 26090 26135 26170 26185 26190 26240 26255 26260 26265 26275 26287 26300 26305 26320 26325 26335 26340 26425 26428 41400 41405 26438 61285 26440 26445 26453 26465 26475 26495 26565 26665 26695 61290 26705 26710 26715 26720 26740 26750 QUIAGEL PG QUIBRON QUIBRON BIDCAP QUIBRON ELIXIR QUINAGLUTE QUINAMM QUINATIME QUINE QUINIDEX EXTENTAB QUINIDINE QUINIDINE GLUCONATE QUINDINE SULFATE QUININE SULFATE QUINOLOR COMPOUND QUIPHILE RABIES VACCINE RAUDIXIN RAUTRAX RAUWOLFIA RAUZIDE REDISOL REGLAN REGONOL REGROTON REGUL-AIDS REHYDRALYTE RELA RELAXADON RENACIDIN RENALGIN RENESE RENESE-R RESERPINE RESERPINE/HYDRALAZINE/HCTZ RESICORT HYDROCORTISONE RESPAIRE RESPBID RESPINOL LA RESPINOL-G RESPIROL RESTORIL RETICULEX RETIN-A RHEABAN RHINIDRIN RHUS TOX ANTIGEN RID RIDAURA RIFADIN RIFAMATE RIFAMPIN RIMACTANE RIOPAN RIOPAN PLUS 26760 26761 26785 26790 26795 26800 26805 26825 26830 26835 26840 26845 26850 26855 26860 26865 26870 61295 61300 26885 26890 26905 26930 26935 26940 26945 41420 27045 27050 27060 27065 27080 27085 27103 27130 27175 27180 27190 27195 27210 27225 27320 27335 27340 27345 27368 27415 27420 27423 61315 27435 27440 27459 27498 RITALIN RITODRINE ROBAXIN ROBAXISAL ROBICILLIN VK ROBIMYCIN ROBINUL ROBITET ROBITUSSIN ROBITUSSIN A-C SYRUP RUBITUSSIN-CF ROBITUSSIN-CF SYRUP ROBITUSSIN-DAC SYRUP ROBITUSSIN-DM COUGH CALMERS ROBITUSSIN-DM SYRUP ROBITUSSIN-PE SYRUP ROCALTROL ROCEPHIN ROFERON-A ROGENIC ROLAIDS ROMILAR RONDEC RONDEC-DM RONDOMYCIN RONIACOL ROXANOL RU-TUSS RU-TUSS EXPECTORANT RU-TUSS W/HYDROCODONE RU-VERT RUBESOL RUBRAMIN PC RUFEN RUTIN RYNA-C RYNA-CX RYNATAN RYNATUSS S.A.S.-500 S.T. 37 SALETO SALICYLAMIDE SALICYLATE SALICYLIC ACID SALINE SALURON SALUTENSIN SANDIMMUNE SANDOPAK SANDOGLOBULIN SANOREX SANSERT SARNA SATRIC 27525 27545 27625 27635 27650 27660 27665 61330 27708 61340 27725 27730 27735 41440 27785 27820 27835 27840 27845 27855 27865 27890 27900 27905 27928 27950 27960 27965 27978 27985 27995 28015 28020 28030 28040 28045 28080 28085 28100 28135 28138 28145 28155 28165 28200 28205 28215 28258 28270 28285 28295 28350 28358 35580 SCHAMBERG LOTION SCOPOLAMINE SEBIZON LOTION SEBULEX SECONAL SECRAN SECRAN PRENATAL SECTRAL SEDATIVE SELDANE SELENIUM SELSUN SELSUN BLUE SENECOT SENOKOT SEPP ANTISEPTIC APPLICATOR SEPTRA SEPTRA DS SER-AP-ES SERAX SERENTIL SERPASIL SERPASIL-APRESOLINE SERPASIL-ESIDRIX SHEPARD'S CREAM LOTION SIBLIN SIGTAB SILAIN-GEL SILICONE SKIN SPRAY SILVADENE SILVER NITRATE SILVER SULFADIAZINE SIMAAL GEL SIMETHICONE SIMPLE SYRUP SIMRON SINEMET SINEQUAN SINGLET SINUBID SINUFED SINULIN SINUS SINUTAB SK-AMITRIPTYLINE SK-AMPICILLIN SK-APAP W/CODEINE SK-DOXYCYCLINE HYCLATE SK-LYGEN SK-PENICILLIN VK SK-PRAMINE SKELAXIN SKIN PREPARATION SLO-BID 28365 28375 28380 41470 28390 28399 28430 28495 28510 28560 28595 28610 28660 28675 28683 28685 28734 28745 28755 28760 28790 28810 28825 28815 28820 28833 28835 28840 28880 28905 28910 28920 29015 29038 29045 41505 29075 29078 29240 41510 29245 29250 29255 29275 29315 29328 29365 29420 29435 29498 29505 29533 29565 29575 SLO-PHYLLIN SLO-PHYLLIN GYROCAP SLO-PHYLLIN SYRUP SLOW FE SLOW-K SMZ SODESTRIN SODIUM CHLORIDE SODIUM CHLORIDE (PHYSIOLOGICAL SALINE) SODIUM CHLORIDE IRRIGATION SODIUM FLUORIDE SODIUM HYPOCHLORITE SODIUM PERBORATE SODIUM SALICYLATE SODIUM SULFACETAMIDE OPHTH SODIUM SULFATE SOLAQUIN SOLATENE SOLFOTON SOLGANAL SUSPENSION SOLU-CORTEF SOLUREX SOMA SOMA COMPOUND SOMA COMPOUND W/CODEINE SOMINEX SOMLYN W/PHENOBARBITAL SOMOPHYLLIN SOPOR SORBIDE T.D. SORBITOL SORBITRATE SPARINE SPASMOLYTIC AGENT SPASQUID ELIXIR SPECTAZOLE SPECTRO-BIOTIC SPECTROBID SPIRITEX SPIRON W/HCTZ SPIRONAZIDE SPIRONOLACTONE SPIRONOLACTONE W/HYDROCHLOROTHIAZIDE SSKI STAPHCILLIN STATICIN STELAZINE STERAMINE STERAPRED STEROID(S) STILBESTROL STOOL SOFTENER STREPTOMYCIN STRESS-VITES 29580 41550 29585 61380 29615 29655 29705 29710 29720 29725 29760 29780 29785 29795 29800 29805 29810 29815 61385 29825 41570 29830 29843 29840 29844 29850 29860 29865 29870 29888 29895 29898 29900 29925 29955 29998 61390 30015 30030 30035 30100 61395 30155 30175 30195 30235 61400 30255 30302 30305 30309 30329 30330 30335 STRESSCAPS STRESSTABS W/IRON STRESSTABS 600 STUART PRENATAL STUARTNATAL 1+1 SUCARYL SUDAFED SUDAFED PLUS SUDAFED S.A. SUDAFED SYRUP SUFEDRIN SULADYNE SULAMYD SODIUM SULF-10 SULFA VAGINAL SULFACEL-15 SULFACET-R LOTION SULFACETAMID SULFACETAMIDE SODIUM SULFADIAZINE SULFAIR SULFALAR SULFAMETHOX W/TRIMETHOPRIM SULFAMETHOXAZOLE SULFAMIDE OPHTHALMIC SULFANILAMIDE SULFAPYRIDINE SULFASALAZINE SULFASOX SULFATRIM SULFEM SULFINPYRAZONE SULFISOXAZOLE SULFONAMIDES DUPLEX SULFUR SULINDAC SULPRED SULTRIN SUMSCREEN SUMYCIN SUPER B COMPLEX W/C LIVER IRON & B-12 SUPROL SURBEX SURBEX-T SURFAK SURMONTIL SUS-PHRINE SUSTAIRE SYMADINE SYMMETREL SYMPATHOMIMETC AGENT SYNACORT SYNALAR SYNALGOS 30340 30365 30370 30395 30398 30400 30468 30475 30480 30485 30495 30513 30535 61405 30545 30553 30555 30565 30575 30585 30590 30595 30630 61415 61420 30650 30655 30660 30670 30690 30723 61435 30725 30730 30735 30740 30745 30755 30756 61440 41595 30760 61445 30775 30777 30781 61450 30782 30790 30795 30805 30810 30815 30820 SYNALGOS-DC SYNEMOL SYNKAYVITE SYNTHROID SYNTHROX SYNTOCINON T-STAT TABRON TACARYL TACE TAGAMET TALACEN TALWIN TAMBOCOR TAMINE TAMOXIFEN TANDEARIL TANNIC ACID TAO TAPAZOLE TAR TARACTAN TAVIST TAZIDIME TEARFAIR TEARISOL TEARS NATURALE TEARS PLUS TEDRAL TEDRAL SA TEGAMIDE TEGISON TEGOPEN TEGRETOL TEGRIN TELDRIN TELEPAQUE TEMARIL TEMAZEPAM TEMOVATE TEMPO TEMPRA TEN-K TENAX TENEX TENORETIC 100 TENORETIC 50 TENORMIN TENUATE TEPANIL TERBUTALINE TERFONYL TERPHAN ELIXIR TERPIN HYDRATE 30825 30835 30850 30870 30880 30915 31000 31020 31045 31050 31058 31075 31085 31093 31120 31140 41610 31145 31165 31175 31200 31205 31215 31225 31230 31235 31240 31258 31260 31263 31265 31275 31324 31325 31330 31335 31365 31390 31395 31400 31410 31428 31430 31440 31455 31460 31483 31485 31508 31543 31515 31542 31545 31550 TERPIN HYDRATE & CODEINE ELIXIR TERPIN HYDRATE ELIXIR TERRAMYCIN TES-TAPE TESSALON PERLE TESTOSTERONE TETANUS ANTITOXIN TETRA TETRACYCLINE TETRACYCLINE HCL TETRAHYDROZOLINE HCL TETREX TEXACORT THALITONE THEO-DUR THEO-ORGANIDIN THEO-24 THEOBID THEOCLEAR THEODRINE THEOLAIR THEOLATE THEOPHED THEOPHYL THEOPHYLLIN ELIXIR THEOPHYLLINE THEOPHYLLINE COMPOUND THEOPHYLLINE EPHEDRINE HYDROXYZINE THEOPHYLLINE-EPHEDRINE-PHENOBARBITAL THEOPHYLLINE/GUAIFENESIN THEOSPAN THEOVENT THERA-GESIC BALM THERAGRAN THERAGRAN HEMATINIC THERAGRAN-M THERAPADS THERAPEUTIC MULTIVITAMIN THERAPEUTIC VITAMIN THERAPEUTIC VITAMIN & MINERAL THERAVIT HEMATINIC THEX FORTE THI-CIN THIACIDE THIAMINE THIAMINE HCL ELIXIR THIOCYL THIODYNE THIORIDAZINE THIORIDAZINE THIOSULFIL THIOTHIXENE THIURETIC THORAZINE 31580 31588 31600 31615 31620 31630 31635 41905 31655 31659 31658 31660 31670 31695 31715 31725 31728 31740 61475 31750 31755 31760 31770 31775 31780 61480 31790 31810 61490 31825 61495 31835 31850 61505 31870 31900 31925 31933 31938 31939 31940 31945 31995 31997 32013 32049 32065 61515 41670 32110 32140 41680 32150 32155 THROAT DISC THROAT PREPARATION THYMOL THYRAR THYRO-TERIC THYROID THYROLAR THYROXINE TIGAN TIMOLIDE TIMOLOL TIMOPTIC TINACTIN TINVER LOTION TITAN TOBRAMYCIN TOBREX OPTHALMIC TOFRANIL TOLAZAMIDE TOLAZOLINE TOLBUTAMIDE TOLECTIN TOLFRINIC TOLINASE TOLMETIN TOLNAFTATE TONACON TONELAX TONOCARD TOPIC TOPICORT TOPICYCLINE TORECAN TORNALATE TOTACILLIN TRAC TRANCOPAL TRANQUILIZER TRANSDERM-NITRO TRANSDERM-SCOP TRANTOIN TRANXENE TRAVERT 10% IN SODIUM CHLORIDE 0.9% TRAZODONE TRENTAL TRI STATIN TRI-HYDROSERPINE TRI-LEVLEN TRI-NORINYL TRI-PHEN-CHLOR TRI-VI-FLOR TRI-VI-FLOR W/IRON TRIACET TRIACIN 32175 32185 32190 32195 32200 32210 32245 32215 32220 32225 32235 32255 32265 32273 61625 32290 32298 32300 32340 32345 32350 32353 32355 32360 32363 32390 32393 32395 32405 32422 35590 32438 32423 35595 32430 32433 32443 32460 32490 32495 32505 32515 32530 61535 32540 32550 32558 32555 TRIAFED TRIAFED SYRUP TRIAM TRIAMCINOLONE TRIAMCINOLONE ACETONIDE TRIAMCINOLONE NYSTATIN TRIAMINIC TRIAMINIC DM TRIAMINIC EXPECTORANT TRIAMINIC EXPECTORANT DH TRIAMINIC INFANT DROPS TRIAMINICIN TRIAMINICOL SYRUP TRIAMTERENE TRIAMTERENE W/HCTZ TRIAVIL TRIBIOTIC OPHTHALMIC TRICHLORMETHIAZIDE TRICONOL TRIDESILON TRIDIHEXETHYL CL 25MG MEPROBAMATE 200 TRIDIL TRIDIONE TRIETHANOLAMINE TRIFLUOPERAZINE TRIHEXYPHENIDYL TRIKATES TRILAFON TRILISATE TRIMCAPS TRIMETHOBENZAMIDE HCL TRIMETHOPRIM TRIMETHOPRIM W/SULFASOXAZOLE TRIMETHOPRIM/SULFAMETHOXAZOLE TRIMOX TRIMPEX TRINALIN REPETABS TRINSICON TRIPELENNAMINE TRIPHED TRIPHENYL TRIPLE ANTIBIOTIC TRIPLE SULFA TRIPLE SULFA VAGINAL TRIPLE VITAMIN DROPS TRIPROLIDINE TRIPROLIDINE PSEUDOEPHED W/CODEINE TRIPROLIDINE W/PSEUDOEPHEDRINE 32580 32610 32625 32660 32665 TRISORALEN TRIVITAMIN DROPS TROCAL TRYPTOPHAN TRYSUL VAGINAL 32670 32710 32720 32735 32767 32770 32790 32805 32810 32825 32830 32835 32845 32840 32855 32870 32880 32890 32905 32910 32915 32920 32925 32930 32935 32945 32955 32990 33068 33073 33105 41730 33149 33130 33140 53148 33148 33155 41745 33160 33170 33190 33215 33225 33240 33250 33275 33280 33290 33300 33330 33355 33375 33405 TU-CILLAN TUCKS TUINAL TUMS TUSS ALLERGINE TUSS-ORNADE TUSSANIL EXPECTORANT TUSSAR TUSSAR-2 SYRUP TUSSEND TUSSEND EXPECTORANT TUSSEX COUGH SYRUP TUSSI-ORGANIDIN TUSSI-ORGANIDIN DM TUSSIONEX TUZON TWIN-K TWO-DYNE TYLENOL TYLENOL NO. 1 TYLENOL NO. 2 TYLENOL NO. 3 TYLENOL NO. 4 TYLENOL W/CODEINE TYLENOL W/CODEINE ELIXIR TYLOX TYMPAGESIC TYZINE ULTRACEF ULTRALENTE INSULIN UNDECYLENIC ACID COMPOUND UNI LOM UNI NATAL PLUS UNIBASE UNICAP UNIFAST UNICELLES UNILAX UNIPHEN UNIPHYL UNIPRES UNISOM URACEL UREA URECHOLINE UREX URIDON URINARY ANTISEPTIC URISED URISEP URISPAS URITIN UROBIOTIC-250 UROLOGIC G UTICORT 33410 33425 33430 33448 33478 33480 33550 33555 33570 33573 33574 35600 33575 33585 33588 33600 33625 33670 33677 33685 33690 33700 33705 33710 33713 33750 61565 33760 33780 33805 33808 33843 33858 33895 33903 33965 33970 33975 33980 33995 34010 34085 34090 34110 34115 34120 34125 34130 34145 34160 34210 34215 34220 34235 UTIMOX V-CILLIN V-CILLIN K VACCINATION VAGINAL SULFA VAGISEC VALISONE VALIUM VALPIN 50 VALPROIC ACID VALRELEASE VANCENASE VANCERIL VANCOCIN HCL VANCOMYCIN VANOXIDE VAPONEFRIN VASELINE VASERETIC VASOCIDIN VASOCLEAR VASOCON VASOCON-A VASODILAN VASODILATOR VASOSULF VASOTEC VASOTRATE SUBLINGUAL VEETIDS VELOSEF VELOSULIN VENTOLIN VERAPAMIL VERMOX VERSACAPS VI-DAYLIN VI-DAYLIN DROPS VI-DAYLIN F VI-DAYLIN F ADC DROPS VI-DAYLIN F PLUS IRON DROPS VI-DAYLIN PLUS IRON VIBRA VIBRAMYCIN VICODIN VICON VICON FORTE VICON-C VICON-PLUS VIGRAN VINCRISTINE VIOFORM VIOFORM-HYDROCORTISONE VIOKASE VIRA-A 34240 34245 34248 34250 34255 42228 34260 34263 41765 34265 34268 34270 34275 34280 34285 34290 34293 34295 34300 34305 34310 61585 34315 34320 34325 34330 34335 34340 34345 34350 34355 34360 00005 34375 00005 34380 34385 34390 00005 34400 34405 34410 34415 34420 03250 34430 34435 34440 34445 03275 34455 34460 34465 34470 VIRILON VIROMED VIROPTIC VISALENS WETTING VISCULOSE VISIDEK II VISINE VISKEN VIST-ED VISTACON VISTAJECT VISTARIL VISTAZINE VISTRAX VITA IRON VITA TOT VITA-FLOR VITA-GLEN VITA-KAPS VITA-METRAZOL VITABEE VITACARN VITACEE VITACEE SYRUP VITADEX-B VITADYE VITAGETT VITAKAPS-M VITAL VITALITY-E VITALYNE VITAMIN A VITAMIN A & D VITAMIN A & D HI-POTENCY VITAMIN A + VITAMIN D VITAMIN A NATURAL VITAMIN A NATURAL NEO-VADRIN VITAMIN A PALMITATE VITAMIN A PLUS D VITAMIN A SOLUBILIZED VITAMIN A SOLUBLE VITAMIN A SOLUBLE NEO-VADRIN VITAMIN A SYNTHETIC VITAMIN A WATER SOLUBLE VITAMIN B COMPLEX VITAMIN B COMPLEX B-12 W/C VITAMIN B COMPLEX ELIXIR VITAMIN B COMPLEX HI POTENCY VITAMIN B COMPLEX W/B-12 VITAMIN B COMPLEX W/VITAMIN C VITAMIN B-1 VITAMIN B-1 & B-12 VITAMIN B-1 & B-12 TONIC VITAMIN B-1 & B-12 W/IRON 34475 34480 34485 34490 34495 34500 61590 34505 34510 34515 34520 34525 34530 34535 34540 34545 34550 34555 34560 34565 34570 34575 34580 34585 34590 34595 34600 34605 34610 34618 34615 34620 34623 08350 08355 34630 34635 34636 34640 34641 34642 61595 34643 34645 34650 34655 34660 34665 34670 34673 34675 34680 34685 34690 VITAMIN B-1 ELIXIR VITAMIN B-1 NEO-VADRIN VITAMIN B-1 W/B-6 VITAMIN B-1 W/B-6 & B/12 VITAMIN B-12 VITAMIN B-12 CRYSTALLINE VITAMIN B-12 W/THIAMINE HCL VITAMIN B-2 VITAMIN B-6 VITAMIN B-6 NEO-VADRIN VITAMIN C VITAMIN C & E VITAMIN C CRYSTAL VITAMIN C FLAVORED VITAMIN C NEO-VADRIN VITAMIN C PLUS E VITAMIN C SYRUP VITAMIN C T.O. VITAMIN CHEWABLE CHILDREN'S VITAMIN D VITAMIN D-2 IN OIL VITAMIN E VITAMIN E & C VITAMIN E NATURAL VITAMIN E NATURAL NEO-VADRIN VITAMIN E NATURAL SOLUBLE VITAMIN E NEO-VADRIN VITAMIN E SKIN OIL VITAMIN E SYNTHETIC VITAMIN E W/SELENIUM VITAMIN E WAFER VITAMIN E ZESTAB VITAMIN K VITAMIN (S) VITAMINS AND MINERALS VITANATE VITANATE FA VITANEED VITAPHEN VITASOL VITAZIN VITEC VITERNA VITERRA VITERRA C VITERRA HIGH POTENCY VITERRA RDA VITERRA RDA PLUS IRON VITERRA-E VITORMAINS VITRON-C VITRON-C PLUS VIVACTIL VIVARIN 34693 34695 34700 34698 34705 34710 34715 34720 34725 34730 34735 34740 34745 34748 34750 34755 34765 34760 34991 34992 34993 34770 42248 61600 34775 34780 34785 34790 34795 34800 34805 34810 34815 34820 34825 34830 34835 61605 34838 41770 34839 34840 34845 34850 34855 34856 34857 34858 34860 34865 34870 34873 34875 34880 VIVIKON VIVONEX VLEM-DOME LIQUID CONCENTRATE VLEMASQUE VLEMINOKX' SOLUTION VOCALZONES VOLAXIN,MODIFIED VOLEX IN SODIUM CHLORIDE VONCE SHAMPOO VONTROL VORAMIL VOSOL VOSOL HC VOXIN VULVAELINE VYTONE W-T LOTION W.D.D. W/W-ANTI-SPAS W/W-FED W/W-HISTINE WAMPOCAP WANS SUPPRETTE WANS SUPPRETTE NO.2 WARFARIN WART OFF WASH 'N DRI TOWELETTE WATER DISTILLED WATER FOR INJECTION WATER FOR IRRIGATION WATER PURIFIED WEHAMIWE WEHDRYL WEHGEN WEHLESS WEHVERT WEIGHTROL WELLBUTRIN WELLCORTIN WELLCOVORIN WES B/C WESCOMEX SURGICAL SCRUB WESLAX WESTADOME WESTCORT WESTHROID WESTRIM WESTVITE WET N SOAK WETTING & SOAKING WETTING SOLUTION BARNES HIND WEXAPHOS WHEAT GERM OIL WHITE PINE COMPOUND 34885 34888 34890 34893 34895 34900 34905 34910 34915 34920 34925 34928 34930 34935 34940 34945 34950 34955 34965 34970 34975 34980 34985 34990 35603 35604 34995 35000 35005 35008 35010 35015 35020 42258 35023 35025 35030 35035 35040 35045 35050 35055 35060 35065 35070 35075 35080 35085 41775 35090 35095 35100 35105 35110 WHITFIELD S WIBI LOTION WIGRAINE WIGRETTES WILD CHERRY SYRUP WINE WINGEL WINSTROL WINTERGREEN OIL WITCH HAZEL WOLFINA WOMAGEN WOOD ALCOHOL WUN-TABS WUN-TABS W/IRON WYAMINE SULFITE WYAMYCIN WYANOID WYANOIDS HC WYCILLIN WYCILLIN INJECTION & PROBENECID TABLET WYDASE WYGESIC WYNOX WYTENSIN WYVAC X SEB H.C. SCALP LOTION X SEB SHAMPOO X SEB T SHAMPOO X-CAINE X-DTAG X-PREP X-PREP BOWEL EVACUANT KIT X-SEB XANAX XERAC AC XERAC BP10 XERAC BP5 XERAC GEL XERO-LUBE XEROFOAM DRESSING XYLO-PFAN XYLOCAINE XYLOCAINE FLAVORED XYLOCAINE HCL XYLOCAINE HCL W/DEXTROSE XYLOCAINE HCL W/EPINEPHRINE XYLOCAINE VISCOUS XYLOCAINE W/GLUCOSE XYLOMET SPRAY XYLOMETAZOLINE XYLOSE CP YEAST YELLOW FEVER VACCINE 35113 35115 35120 35123 35130 35135 35138 35125 35140 35145 35148 41780 35150 35155 35160 35165 35170 35175 35180 35185 35190 35195 35200 35205 35210 35215 35220 35225 35230 35233 35235 41785 35240 35240 35240 41790 35245 35250 35255 41795 35260 35265 35270 35275 35280 42263 35285 35290 35295 35300 35305 35310 35313 35315 YF-VAX YODOXIN YOHIMBINE YUTOPAR Z-BEC Z-PRO-C Z-TEC Z.B.T. BABY POWDER ZACTANE ZACTIRIN ZANOSAR ZANTAC ZARONTIN ZAROXOLYN ZARUMIN ZEASORB MEDICATED ZEM HISTINE ZEMACOL MEDICATED LOTION ZEMACON ZEMALD SKIN CLEANSER ZENI-B W/C ZENIVITES M ZENTINIC ZENTRON ZEPHIRAN CHLORIDE ZESTE ZESTE M.T. ZETAR ZETRAN ZIBA-RX ZIDE ZINACEF ZINC ZINC 15 ZINC 25 ZINC CHLORIDE ZINC GLUCONATE ZINC OXIDE ZINC PASTE (LASSARS) ZINC STEARATE ZINC SULFATE ZINC SULFATE COMPOUND ZINC SULFATE MUROCOLL NO. ZINC SULFATE NEO-VADRIN ZINC SULFIDE CONPOUND LOTION ZINC TRACE METAL ZINC-20 ZINC-220 ZINCATE ZINCFRIN ZINCOFAX ZINCON ZINCTRACE ZIPAN 35320 35323 35325 35330 35335 35338 35340 41880 35341 35342 35343 35344 61610 35345 35350 35355 35360 35365 35370 35375 35380 ZIRADRYL LOTION ZNG ZOLINE-M ZOLINE-S ZOLYSE ZOMAX ZONIUM CHLORIDE ZORPRIN ZOVIRAX ZOXAPHEN ZS-220 ZWOLFE ZYDONE ZYLAN ZYLOPRIM ZYMACAP ZYMALIXIR ZYMASYRUP ZYMAVITES ZYMENOL ZYMME GENERIC DRUG NAMES AND CODES The system adopted to process data on generic drugs was originally developed by the National Center for Health Statistics (Koch, 1980) as part of the National Ambulatory Care Survey (NAMCS). 50008 50005 50020 50025 50030 50035 50060 50078 50088 50090 50103 50105 50113 50130 50140 50145 50163 50165 50175 50180 50182 50183 ACEBUTOLOL HYDROCHLORIDE ACETAMINOPHEN ACETAZOLAMIDE ACETIC ACID ACETOHEXAMIDE ACETONE ACETYLCYSTEINE ACYCLOVIR ALBUTEROL ALCOHOL ALLANTOIN ALLOPURINOL ALPRAZOLAM ALUMINUM ACETATE ALUMINUM CHLORIDE ALUMINUM HYDROXIDE ALUMINUM SULFATE AMANTADINE AMCINONIDE AMIKACIN AMILORIDE AMINACRINE 50185 50190 50195 50200 50210 50223 50230 50245 50250 50258 50260 50265 50270 50275 50283 50290 50300 50305 50310 50325 50338 50410 50418 50420 50430 50433 50435 50440 50445 50447 50450 50455 50460 50490 50495 50499 50500 50508 50515 50520 50530 50535 50538 50540 50545 50550 50555 50565 50570 50575 50580 50585 50588 50595 AMINO ACIDS AMINOACETIC ACID AMINOBENZOIC ACID AMINOCAPROIC ACID AMINOPHYLLINE AMIODARONE HCL AMITRIPTYLINE AMMONIUM CHLORIDE AMOBARBITAL AMOXAPINE AMOXICILLIN AMPHETAMINE AMPHOTERICIN AMPICILLIN AMYLASE ANISE OIL ANISOTROPINE ANTAZOLINE ANTHRALIN ANTIPYRINE ANTIVENIN BEE STING ASPIRIN ATENOLOL ATROPINE ATTAPULGITE AURANOFIN AUROTHIOGLUCOSE AZATADINE AZATHIOPRINE BACAMPICILLIN BACITRACIN BACLOFEN BALSAM BECLOMETHASONE BELLADONNA BENACTYZINE BENDROFLUMETHIAZIDE BENTONITE BENZALKONIUM CHLORIDE BENZETHONIUM CHLORIDE BENZOCAINE BENZOIC & SALICYLIC ACID BENZOIC ACID BENZOIN BENZONATATE BENZOYL PEROXIDE BENZPHETAMINE BENZTROPINE BENZYL ALCOHOL BENZYL BENZOATE BETA-CAROTENE BETAMETHASONE BETAXOLOL HCL BETHANECHOL 50613 50615 50620 50625 50630 50635 50638 50632 50640 50653 50660 50663 50668 50680 50685 50690 50698 50705 50706 50708 50713 50720 50728 50733 50745 50755 50760 50775 50887 50785 50773 50800 50802 50806 50811 50770 50824 50835 50845 50860 50868 50869 50873 50875 50880 50895 50898 50899 50900 50920 50923 50933 50935 50940 BILE SALTS BIOFLAVONOIDS BIOTIN BIPERIDEN BISACODYL BISMUTH ANTI-DIARRHEA AGENTS BISMUTH IODIDE BISMUTH OXIDE BISMUTH SALICYLATE BITOLTEROL MESYLATE BORIC ACID BRAN BRILLIANT BLUE BROMOCRIPTINE BROMODIPHENHYDRAMINE BROMPHENIRAMINE BUCHU BUCLIZINE BUFFERS BUMETANIDE BUSPRIONE HCL BUTABARBITAL BUTALBITAL BUTOCONAZOLE NITRATE CAFFEINE CALAMINE CALCITONIN CALCIUM ACETATE CALCIUM CARBASPIRIN CALCIUM CARBONATE CALCIUM GLYCEROPHOSPHATE CALCIUM HYDROXIDE CALCIUM IODIDE CALCUIM ION CALCIUM PANTOTHENATE CALCIUM REPLACEMENT AGENTS CALJUPUT OIL CALLICREIN CAMPHOR CANTHARIDIN CAPSICUM CAPTOPRIL CARAMIPHEN CARBACHOL CARBAMAZEPINE CARBENICILLIN CARBETAPENTANE CARBIDOPA CARBINOXAMINE CARISOPRODOL CARMELLOSE CASANTHRANOL CASCARA CASTOR OIL 50945 50950 50960 50961 50966 50968 50980 50995 51005 51008 51020 51023 51025 51040 51045 51050 51053 51055 51063 51064 51066 51075 51085 51095 51100 51105 51110 51115 51120 51130 51150 51155 51160 51165 51170 51175 51177 51190 51195 51200 51205 51215 51218 51220 51225 51227 51229 51235 51245 51250 51255 51243 51257 51260 CEFACLOR CEFADROXIL CEFAZOLIN CEFONICID SODIUM CEFTAZIDIME CELLULASE CEPHALEXIN CEPHALOTHIN CEPHRADINE CERESIN WAX CETYL ALCOHOL CETYLPYRIDINIUM CHARCOAL CHLORAL HYDRATE CHLORAMBUCIL CHLORAMPHENICOL CHLORCYCLIZINE CHLORDIAZEPOXIDE CHLORHYDROXYQUINOLONE CHLORIDE ION CHLORMEZANONE CHLOROBUTANOL CHLOROPHYLL CHLOROQUINE CHLOROTHIAZIDE CHLOROTHYMOL CHLOROTRIANISENE CHLOROXINE CHLOROXYLENOL CHLORPHENIRAMINE CHLORPROMAZINE CHLORPROPAMIDE CHLORPROTHIXENE CHLORTETRACYCLINE CHLORTHALIDONE CHLORZOXAZONE CHOLECALCIFEROL CHOLESTYRAMINE CHOLINE CHOLINE SALICYLATE CHORIONIC GONADOTROPIN CHYMOTRYPSIN CICLOPIROX CIMETIDINE CINNAMON OIL CINOXACIN CIPROFLOXACIN HCL CITRIC ACID CLEMASTINE CLIDINIUM CLINDAMYCIN CLOBETASOL PROPIONATE CLOCORTOLONE CLOFIBRATE 51265 51270 51275 51280 51290 51300 51305 51308 51340 51345 51355 51360 51375 51380 51393 51400 51405 51410 51415 51430 51435 51440 51443 51445 51460 51465 51470 51480 51485 51493 51510 51518 51540 51545 51550 51555 51575 51585 51590 51595 51598 51599 51600 51605 51615 51620 51625 51633 51635 51638 51640 51645 51665 51670 CLOMIPHENE CLONAZEPAM CLONIDINE CLORAZEPATE CLOTRIMAZOLE CLOXACILLIN COAL TAR COBALAMIN CODEINE COLCHICINE COLESTIPOL COLISTIN COLLODION COMBINATION PRODUCT COPPER CORTICOTROPIN CORTISONE COSYNTROPIN COTTONSEED OIL CROMOLYN SODIUM CROTAMITON CRYPTENAMINE CUBEB CUPRIC SULFATE CYCLACILLIN CYCLANDELATE CYCLOBENZAPRINE CYCLOPENTOLATE CYCLOPHOSPHAMIDE CYCLOSPORINE CYPROHEPTADINE CYSTINE DANAZOL DANTHRON DANTROLENE DAPSONE DEFEROXAMINE DEHYDROCHOLIC ACID DEMECARIUM DEMECLOCYCLINE DEOXYRIBONUCLEASE DEQUALINIUM ACETATE DESERPIDINE DESIPRAMINE DESMOPRESSIN DESONIDE DESOXIMETASONE DETERGENT DEXAMETHASONE DEXBROMPHENIRAMINE DEXCHLORPHENIRAMINE DEXPANTHENOL DEXTROAMPHETAMINE DEXTROMETHORPHAN 51675 51685 51695 51705 60015 51709 51715 51720 51725 51730 51735 51740 51750 51755 51760 51763 51770 51775 51780 52647 51785 51790 51800 51803 51810 51817 51903 51860 51865 51868 51913 51915 51920 51925 51927 51935 51955 51960 51965 51970 52000 52005 52008 52015 52023 52028 52024 52035 52040 52045 52048 52050 52055 52060 DEXTROSE DEXTROTHYROXINE DIAZEPAM DIBUCAINE DICHLORALANTIPYRINE DICHLORODIFLUOROMETHANE DICHLORPHENAMIDE DICLOXACILLIN DICUMAROL DICYCLOMINE DIENESTROL DIETARY SUPPLEMENT DIETHYLPROPION DIETHYLSTILBESTROL DIFLORASONE DIACETATE DIFUNISAL DIGITALIS DIGITOXIN DIGOXIN DIHYDROCODEINE DIHYDROERGOIAMINE DIHYDROTACHYSTEROL DIHYDROXYALUMINUM SODIUM CARBONATE DILTIAZEM DIMENHYDRINATE DIMETHICONE DIPH PERTUSSIS TETANUS VACCINE DIPHENHYDRAMINE DIPHENIDOL DIPHENOXYLATE DIPIVEFRIN DIPYRIDAMOLE DISOPYRAMIDE DISULFIRAM DIVALPROEX SODIUM DOCUSATE DOXEPIN DOXORUBICIN DOXYCYCLINE DOXYLAMINE DYPHYLLINE ECHOTHIOPHATE ECONAZOLE EDETATE DISODIUM ELECTROLYTES ENALAPRIL ENCAINIDE HCL EPHEDRINE EPINEPHRINE ERGOCALCIFEROL ERGOLOID MESYLSTES ERGONOVINE ERGOTAMINE ERYTHRITYL TETRANITRATE 52065 52070 52075 52080 52082 52085 52090 52095 52100 52105 52168 52120 52125 52130 52135 52145 52155 52185 52169 52190 52195 52198 52215 52220 52270 52275 52278 52295 52303 52304 52305 52310 52318 52320 52325 52330 52335 52345 52350 52353 52355 52358 52360 52370 52380 52385 52395 52398 52400 52405 52417 52422 52430 62435 ERYTHROMYCIN ESTRADIOL ESTROGENS ESTRONE ESTROPIPATE ETHACRYNIC ACID ETHAMBUTOL ETHAVERINE ETHCHLORVYNOL ETHER ETHNODIOL DIACETATE ETHOHEPTAZINE ETHOPROPAZINE ETHOSUXIMIDE ETHOTOIN ETHOXZOLAMIDE ETHYL CHLORIDE ETIDRONATE DISODIUM ETRETINATE EUCALYPTOL EUGENOL FAMOTIDINE FENFLURAMINE FENOPROFEN FIBRINOLYSIN FLAVOXATE FLECAINIDE ACETATE FLUDROCORTISONE FLUMETHIAZIDE FLUNISOLIDE FLUOCINOLONE FLUOCINONIDE FLUORIDE FLUOROMETHOLONE FLUOROURACIL FLUOXYMESTERONE FLUPHENAZINE FLURANDRENOLIDE FLURAZEPAM FLURBIPROFEN SODIUM FOLIC ACID FOOD SUPPLEMENT FORMALDEHYDE FRUCTOSE FURAZOLIDONE FUROSEMIDE GELATIN GEMFIBROZIL GENTAMICIN GENTIAN VIOLET GLIPIZIDE GLUCONIC ACID GLUCOSE ENZYMATIC TEST GLUTAMIC ACID 52445 52447 52450 52455 52470 52478 52480 52485 52490 52492 52493 52495 52498 52500 52503 52505 52510 52520 52525 52535 52533 52540 52545 52550 52560 52595 52600 52610 52615 52619 52630 52645 52650 52655 52660 52665 52670 52675 52685 52690 52691 52700 52710 52715 52720 52723 52730 52745 52750 52753 52770 52775 52780 52752 GLUTETHIMIDE GLYBURIDE GLYCERIN GLYCOPYRROLATE GOLD SODIUM THIOMALATE GRAMICIDIN GRISEOFULVIN GUAIACOL GUAIFENESIN GUANABENZ GUANARDEL GUANETHIDINE GUANFACINE HCL GUANIDINE HALAZEPAM HALAZONE HALCINONIDE HALOPERIDOL HALOPROGIN HAMAMELIS WATER HEMICELLULASE HEPARIN HEPATITIS B IMMUNE GLOBULIN HESPERIDIN HEXACHLOROPHENE HEXYLRESORCINOL HISTAMINE HISTOPLASMIN HOMATROPINE HOREHOUND HYDRALAZINE HYDROCHLOROTHIAZIDE HYDROCODONE HYDROCORTISONE HYDROFLUMETHIAZIDE HYDROGEN PEROXIDE HYDROMORPHONE HYDROQUINONE HYDROXYAMPHETAMINE HYDROXYCHLOROQUINE HYDROXYETHYLCELLULOSE HYDROXYPROPYL METHYLCELLULOSE HYDROXYUREA HYDROXYZINE HYOSCYAMINE HYOSCYAMUS IBUPROFEN IMIPRAMINE IMMUNE GLOBULIN INDAPAMIDE INDOMETHACIN INFANT FORMULA INFLUENZA VIRUS VACCINE INHALER 52785 52790 52791 52795 52800 52825 52830 52820 52845 52847 52850 52855 52870 52883 52890 52885 52895 52900 52908 52915 52920 52925 52927 52930 52935 52943 52945 52948 52950 52955 52972 52978 52973 52979 52975 52980 52985 52986 52995 53003 53004 53010 53020 53035 53043 53050 53055 53065 53070 53090 53095 53105 53110 53115 INOSITOL INSULIN INTERFERON ALFA-2A INULIN INVERT SUGAR IODINATED GLYCEROL IODINE SOLUTIONS IODINE TOPICAL PREPARATIONS IODOCHLORHYDROXYQUIN IODOFORM IODOQUINOL IOPANOIC ACID IPECAC IPRATROPIUM BROMIDE IRON BILE SALTS IRON PREPARATIONS ISOCARBOXAZID ISOETHARINE ISOMETHEPTENE MUCATE ISONIAZID ISOPROPAMIDE ISOPROPYL ALCOHOL ISOPROPYL PALMITATE ISOPROTERENOL ISOSORBIDE ISOTRENTINOIN ISOXSUPRINE JUNIPER KANAMYCIN KAOLIN KETOCONAZOLE KETOPROFEN L-TRYPTOPHANE LABETALOL HCL LACTASE LACTIC ACID LACTOBACILLUS ACIDOPHILUS LACTOBACILLUS BULGARICUS LACTULOSE LANOLIN LAURETH LAVENDER OIL LECITHIN LEUCOVORIN LEVOBUNOLOL HCL LEVODOPA LEVORPHANOL LEVOTHYROXINE LIDOCAINE LINCOMYCIN LINDANE LINSEED OIL LIOTHYRONINE LIOTRIX 53118 53120 53125 53135 53140 53145 53148 53150 53160 53170 53175 53195 53190 53180 53220 53248 53250 53260 53275 53277 53280 53285 53290 53295 53298 53300 53320 53325 53328 53330 53335 53340 53350 53355 53365 53370 53385 53435 53438 53450 53460 53470 53475 53485 53500 53510 53515 53520 53525 53530 53535 53540 53550 53560 LIPASE LITHIUM LIVER DERIVATIVE LOMUSTINE LOPERAMIDE LORAZEPAM LOVASTATIN LOXAPINE LYSINE MAGALDRATE MAGNESIUM ANTACIDS MAGNESIUM CATHARTICS MAGNESIUM CHLORIDE MAGNESIUM GLUCONATE MAGNESIUM SALICYLATE MAPROTILINE MAZINDOL MEBENDAZOLE MECLIZINE MECLOFENAMATE MEDROXYPROGESTERONE MEDRYSONE MEFENAMIC ACID MEGESTROL MEGLUMINE MELPHALAN MENOTROPINS MENTHOL MENTHYL ANTHRANILATE MEPENZOLATE MEPERIDINE MEPHENESIN MEPHENYTOIN MEPHOBARBITAL MEPREDNISONE MEPROBAMATE MERCAPTOPURINE MESORIDAZINE MESTRANOL METAPROTERENOL METAXALONE METHACYCLINE METHADONE METHAMPHETAMINE METHANTHELINE METHAQUALONE METHARBITAL METHAZOLAMIDE METHDILAZINE METHENAMINE METHICILLIN METHIMAZOLE METHIONINE METHOCARBAMOL 53570 53585 53595 53600 53605 53610 53647 53620 53630 53635 53640 53645 53650 53655 53660 53670 53675 53680 53688 53690 53445 53700 53712 53715 53720 53723 53725 53730 53754 53755 53758 53760 53778 53780 53840 53845 53855 53860 53875 53880 53895 53903 53905 53915 53919 53920 53922 53930 53935 53940 53945 53953 53955 53965 METHOTREXATE METHOXSALEN METHOXYPHENAMINE METHSCOPOLAMINE METHSUXIMIDE METHYCLOTHIAZIDE METHYL NICOTINATE METHYL SALICYLATE METHYLCELLULOSE METHYLDOPA METHYLENE BLUE METHYLERGONOVINE METHYLPARABEN METHYLPHENIDATE METHYLPREDNISOLONE METHYLTESTOSTERONE METHYPRYLON METHYSERGIDE METOCLOPRAMIDE METOLAZONE METOPROLOL METRONIDAZOLE MEXILETINE HCL MICONAZOLE MINERAL OIL MINERALS MINOCYCLINE MINOXIDIL MOMETASONE FUROATE MONOBENZONE MONETHANOLAMINE MORPHINE MOXALACTAM MULTIVITAMINS GENERAL NADOLOL NAFCILLIN NALBUPHINE NALIDIXIC ACID NAPHAZOLINE NAPROXEN NEOMYCIN NETILMICIN NIACIN NIACINAMIDE NICOTINE POLACRILEX NICOTINYL ALCOHOL NIFEDIPINE NITROFURANTOIN NITROFURAZONE NITROGEN NITROGLYCERIN NONIFENSINE MALEATE NONOXYNOL NORETHINDRONE 53969 53970 53975 53990 53995 54000 54015 54020 54030 54035 54050 54065 54070 54080 54085 54100 54105 54113 54094 54115 54120 54140 54095 54148 51450 54155 54160 54165 54175 54190 54205 54215 54220 54227 54228 54238 54240 54245 54250 54260 54268 54275 54290 54295 54297 54300 54305 54310 54315 54320 54330 54332 54350 54365 NORFLOXACIN NORGESTREL NORTRIPTYLINE NYLIDRIN NYSTATIN OATMEAL OINTMENT HYDROPHILIC OLEANDOMYCIN OLIVE OIL OPIUM ORPHENADRINE OX BILE EXTRACT OXACILLIN OXANDROLONE OXAZEPAM OXTRIPHYLLINE OXYBUTYNIN OXYCHOLESTRIN OXYCODONE OXYGEN OXYMETAZOLINE OXYPHENBUTAZONE OXYPHENCYCLIMINE OXYQUINOLONE OXYTERACYCLINE OXYTOCIN PANCREATIN PANCRELIPASE PANTOTHENIC ACID PAPAVERINE PARAMETHASONE PAREGORIC PARGYLINE PASSIFLORA EXTRACT PASSIFLORA EXTRACT PECTIN PEMOLINE PENICILLAMINE PENICILLIN PENICILLIN G PROCAINE PENICILLIN V POTASSIUM PENTAERYTHRITOL PENTAZOCINE PENTOBARBITAL PENTOXIFYLLINE PENTYLENETETRAZOL PEPPERMINT PEPSIN PERMETHRIN PERPHENAZINE PETROLATUM PETROLEUM DISTILLATE PHENACETIN PHENAZOPYRIDINE 54370 54375 54378 54385 54390 54395 54400 54405 54415 54430 54440 54450 54455 54460 54465 54468 54470 54480 54495 54500 54501 54503 54523 54528 54535 54565 54585 54587 54590 54595 54597 54598 54605 54610 54735 54620 54625 54640 54700 54710 54713 54655 54748 54749 54755 54760 54765 54770 54775 54795 54800 54805 54810 54815 PHENDIMETRAZINE PHENELZINE PHENINDAMINE PHENIRAMINE PHENMETRAZINE PHENOBARBITAL PHENOL PHENOLPHTHALEIN PHENOXYBENZAMINE PHENTERMINE PHENYL SALICYLATE PHENYLBUTAZONE PHENYLEPHRINE PHENYLMERCURIC NITRATE PHENYLPROPANOLAMINE PHENYLTOLOXAMINE PHENYTOIN PHOSPHORIC ACID PHYTONADIONE PILOCARPINE PINDOLOL 1 PINE TAR PIPERONYL PIROXICAM PLACEBO POISON IVY EXTRACT POLLEN ANTIGEN POLYCARBOPHIL CALCIUM POLYETHYLENE GLYCOL POLYMIXIN B POLYOXYETHYLENENONYL PHENOL POLYSORBATE POLYTHIAZIDE POLYVINYL ALCOHOL POTASSIUM ACIDIFYING AGENTS POTASSIUM ALKALINIZING AGENTS POTASSIUM AMINOBENZOATE POTASSIUM BITARTRATE POTASSIUM GUAIACOL SULFONATE POTASSIUM IODIDE POTASSIUM ION POTASSIUM REPLACEMENT SOLUTIONS POVIDONE POVIDONE-IODINE PRAMOXINE PRAZEPAM PRAZOSIN PREDNISOLONE PREDNISONE PRIMIDONE PROBENECID PROBUCOL PROCAINAMIDE PROCAINE 54825 54830 54835 54845 54850 54860 54870 54890 54865 54895 54905 54910 54920 54923 54950 54960 54965 54975 54980 54983 54985 54990 54995 55000 55030 55035 55040 55045 55050 55060 55061 55064 55070 55075 55080 55085 55105 55108 55110 55117 55135 55140 55150 55160 55165 55180 55185 55195 55200 55210 55213 55225 55235 55240 PROCARBAZINE PROCHLORPERAZINE PROCYCLIDINE PROGESTERONE PROMAZINE PROMETHAZINE PROPANTHELINE PROPOXYPHENE PROPRANOLOL PROPYLENE GLYCOL PROPYLPARABEN PROPYLTHIOURACIL PROTEIN HYDROLYSATE PROTEINASE PROTRIPTYLINE PSEUDOEPHEDRINE PSYLLIUM PYRANTEL PYRAZINAMIDE PYRETHRINS PYRIDOSTIGMINE PYRIDOXINE PYRILAMINE PYRIMETHAMINE QUINACRINE QUINESTROL QUINETHAZONE QUINIDINE QUININE RABIES VACCINE RACEMETHIONINE RANTIDINE RAUWOLFIA RESCINNAMINE RESERPINE RESORCINOL RIBOFLAVIN RICINOLEIC ACID RIFAMPIN RITODRINE RUTIN SACCHARIN SALICYLAMIDE SALICYLIC ACID SALSALATE SCOPOLAMINE SECOBARBITAL SELENIUM SULFIDE SENNA SESAME OIL SHARK LIVER OIL SILVER NITRATE SILVER SULFADIAZINE SIMETHICONE 55253 55265 55270 55274 55275 55280 55295 55315 55320 55330 55345 55348 55360 55370 55380 55385 55390 55393 56325 55405 55420 55425 55430 55435 55455 55460 55465 55470 55480 55485 55490 55493 55500 55518 55525 55540 55543 55548 55550 55555 55565 55567 55570 55575 55580 55585 55590 55600 55615 55610 55618 55623 55636 55640 SKIN RESP FACTOR YEAST SOAP SODIUM ACETATE SODIUM AMINOBENZOATE SODIUM BENZOATE SODIUM BICARBONATE SODIUM BORATE SODIUM CHLORIDE SODIUM CITRATE SODIUM FLUORIDE SODIUM HYPOCHLORITE SODIUM ION SODIUM LAURYL SULFATE SODIUM NITROPRUSSIDE SODIUM PERBORATE SODIUM PHOSPHATE SODIUM POLYSTYRENE SULFONATE SODIUM PROPIONATE SODIUM SALICYLATE SODIUM SULFATE SODIUM THIOSALICYLATE SODIUM THIOSULFATE SOMATROPIN SORBITOL SPIDER-BITE ANTIVENIN SPIRONOLACTONE STANNOUS FLUORIDE STANOZOLOL STARCH STEARIC ACID STEARYL ALCOHOL STOMACH POWDERED STREPTOMYCIN SUCRALFATE SULFACETAMIDE SULFADIAZINE SULFAMERAZINE SULFAMETHAZINE SULFAMETHIZOLE SULFAMETHOXAZOLE SULFANILAMIDE SULFANILYLBENZAMIDE SULFAPYRIDINE SULFASALAZINE SULFATHIAZOLE SULFINPYRAZONE SULFISOXAZOLE SULFOSALICYLIC ACID SULINDAC SULPHUR SUPROFEN SURFACTANT SYRUP TALBUTAL 55645 55650 55655 55668 56290 55670 55673 55675 55695 55700 55715 55725 55730 55745 55750 55755 55765 55770 55780 55785 55795 55801 55815 55820 55825 55845 55848 55850 55860 55870 55875 55880 55885 55890 55893 55900 55903 55905 55910 55915 55925 55928 55930 55943 55950 55955 55960 55967 55975 55985 55990 56000 55997 56005 TALC TAMOXIFEN TANNIC ACID TEMAZEPAM TERAZOSIN HCL TERBUTALINE TERFENADINE TERPIN HYDRATE TESTOSTERONE TETANUS ANTITOXIN TETRACAINE TETRACYCLINE TETRAHYDROZOLINE THEOPHYLLINE THIABENDAZOLE THIAMINE THIETHYLPERAZINE THIMEROSAL THIOPENTAL THIORIDAZINE THIOTHIXENE THONZONIUM BROMIDE THYMOL THYROGLOBULIN THYROID TIMOLOL TITANIUM TOBRAMYCIN TOCAINIDE HYDROCHLORIDE TOLAZAMIDE TOLAZOLINE TOLBUTAMIDE TOLMETIN TOLNAFTATE TOLU BALSAM TRANYLCYPROMINE TRAZODONE TRETINOIN TRIACETIN TRIAMCINOLONE TRIAMTERENE TRIAZOLAM TRICHLORMETHIAZIDE TRICHLOROFLUROMETHANE TRIDIHEXETHYL CHLORIDE TRIETHANOLAMINE TRIFLUOPERAZINE TRIFLURIDINE TRIHEXYPHENIDYL TRIMEPRAZINE TRIMETHADIONE TRIMETHOBENZAMIDE TRIMETHOPRIM TRIMIPRAMINE 56010 56015 56030 56045 56020 56048 56050 56110 50000 56120 56123 56133 56134 56145 56150 56161 56163 56170 56180 56185 56340 56345 56192 56193 56194 56195 56198 56205 56210 56238 56240 56250 56260 56262 56285 56265 56288 56275 TRIOXSALEN TRIPELENNAMINE TROLAMINE TROPICAMIDE TROPROLIDINE TRYPSIN TRYPTOPHAN UNDECYLENIC ACID UNDETERMINED UREA UROFOLLITROPIN UROLOGIC SOLUTION-G UVA URSI VALPROIC ACID VANCOMYCIN VEGETABLE OIL VERAPAMIL VIDARABINE VINCRISTINE VITAMIN A VITAMIN B VITAMIN B COMPLEX VITAMIN B-12 VITAMIN C VITAMIN D VITAMIN E VITAMIN K WARFARIN WATER STERILE WOOL ALCOHOLS XYLOMETAZOLINE YEAST YOHIMBENE ZEA ZINC SULFATE ZINC TOPICAL AGENTS ZIRCONIUM ZOMEPIRAC T QUESTIONS FROM ROUND 1 OF THE HOUSEHOLD SURVEY T65. some Has (PERSON) ever talked with an insurance agent or found out in other way what it would cost to buy private health insurance for (himself/herself)? Yes...........................1 (T67) No............................2 (T67) T66. out Has (PARENT/GUARDIAN) ever talked with an insurance agent or found in some other way what it would cost to buy private health insurance for (PERSON)? Yes...........................1 No............................2 T67. kind Has (PERSON) ever been refused health insurance, or limited in the of health insurance coverage (he/she) could buy, because of poor health? Yes...........................1 (NP:BOX T12/T68) No............................2 (NP:BOX T12/T68) TECHNICAL SPECIFICATIONS FOR UTILIZATION AND EXPENDITURE VARIABLES The technical specifications for the construction of the person-level utilization and expenditure variables has been provided to the user on this CD-ROM. This will hopefully facilitate a better understanding of the analytic uses of these variables. The user is provided with the following variable information: (1) the event source of the variable; (2) the variable label found on the file; (3) a generic code which explains how the variable was constructed and (4) a conceptual description of the variable. Key for Abbreviations Used in Section T DVIS: a File 1 of Public Use Tape 14.3, where each record corresponds to visit to a dental provider , for each eligible and responding person. EROM: a File 3 of Public Use Tape 14.5, where each record corresponds to visit to a hospital emergency room, regardless of type of provider seen, for each eligible and responding person. HVIS: the File 1 of Public Use Tape 14.2, where each record corresponds to set of formal home health care services provided during the year by each unique type of provider sent by each unique agency, for each eligible and responding person. MEXP: each File 2 of Public Use Tape 14.2, where each record corresponds to unique type of medical item purchased, rented, or otherwise obtained per round, for each eligible and responding person. MVIS: a File 1 of Public Use Tape 14.5, where each record corresponds to visit or telephone call to a physician's office (including HMOs and health departments) in settings other than a hospital or at home, and to other providers of care (e.g., chiropractors and psychologists), for each eligible and responding person. OPAT: a File 2 of Public Use tape 15.5, where each record corresponds to visit to a hospital outpatient department, regardless of type of provider seen, for each eligible and responding person. PMED: each File 1 of Public Use Tape 14.1, where each record corresponds to unique medication purchased or otherwise obtained during a reference period, for each eligible and responding person. STAZ: one File 1 of Public Use tape 14.4, where each record corresponds to hospital stay, for each eligible and responding person. Variable Name: HOSP Event Source(s): STAZ Variable Label: # OF HOSPITAL ADMISSIONS (SET 1) Technical Specifications: Count of STAZ records with NUMNGHTX>=1 Description: Constructed variable indicating number of hospital admissions with a stay of 1 or more nights. In Set 1, all newborns have charges and hospital utilization related to birth (unlike Set 2, which assigns hospitalization and utilization charges to the mother, not the newborn). Variable Name: HOSPX Event Source(s): STAZ Variable Label: # OF HOSPITAL ADMISSIONS (SET 2) Technical Specifications: Count of modified STAZ records w/NUMNGHTX>=1. STAZ records modified for birth of child (normal delivery), child's charges set to 0, sum with mother's charges, mother-baby identified by ODU, PN, MOMPN, EN, and RSNINHOS=4 or 5, BABYNORM=1 and NORMDELV=1. Description: Constructed variable indicating number of hospital admissions with a stay of 1 or more nights. In Set 2, expenditures and hospitalization utilization related to birth for newborns are bundled with the mothers' expenditures and utilization, unless the delivery was abnormal or if the infant's hospital stay exceeded that of the mother's. Variable Name: HOSPNGT Event Source(s): STAZ Variable Label: # OF NIGHTS IN HOSPITAL (SET 1) Technical Specifications: Sum of NUMNGHTX from STAZ records w/NUMNGHTX>=1 Description: Constructed variable indicating the total number of nights in the hospital. In Set 1, stays for newborns are counted separately from mother's stay. Variable Name: HOSPNGTX Event Source(s): STAZ Variable Label: # OF NIGHTS IN HOSPITAL (SET 2) Technical Specifications: Sum of NUMNGHTX from STAZ records w/NUMNGHTX>=1. STAZ records modified for birth of child (normal delivery), child's charges set to 0, sum with mother's charges, mother-baby identified by ODU, PN, MOMPN, EN, and RSNINHOS =4 or 5, BABYNORM=1 and NORMDELV=1. Description: Constructed variable indicating the total number of nights in hospital. In Set 2, stays for newborns are not counted separately from mother's stay unless delivery was abnormal or if the infant's stay exceeded the mother's. Variable Name: HOSMDEXP Event Source(s): STAZ Variable Label: HOSPITAL PHYSICIAN EXPENSE (SET 1) ($) Technical Specifications: Sum of EXPDRX from STAZ records with NUMNGHTX>=1 Description: Constructed variable indicating the hospital physician expense per stay for physicians billing separately for inpatient services. In Set 1, stays for newborns are counted separately from mother's stay. Variable Name: HSXMDEXP Event Source(s): STAZ Variable Label: HOSPITAL PHYSICIAN EXPENSE (SET 2) ($) Technical Specifications: Sum of EXPDRX from STAZ records with NUMNGHTX>=1. STAZ records modified for birth of child (normal delivery), child's charges set to 0, sum with mother's charges, mother-baby identified by ODU, PN, MOMPN, EN, and RSNINHOS=4 or 5, BABYNORM=1 and NORMDELV=1. Description: Constructed variable indicating the hospital facility expense per stay for physicians billing separately for inpatient services. In Set 2, stays for newborns are not counted separately from mother's stay unless delivery was abnormal or if the infant's stay exceeded the mother's. Variable Name: HOSFCEXP Event Source(s): STAZ Variable Label: HOSPITAL FACILITY EXPENSE (SET 1) ($) Technical Specifications: Sum of EXPFACX from STAZ with NUMNGHTX>=1 Description: Constructed variable indicating the hospital facility expense per stay. In Set 1, stays for newborns are counted separately from mother's stay. Expense includes hospital care, room and board, laboratory charges, x-ray, and services provided by physicians not billing separately. Variable Name: HSXFCEXP Event Source(s): STAZ Variable Label: HOSPITAL FACILITY EXPENSE (SET 2) ($) Technical Specifications: Sum of EXPFACX from STAZ records with NUMNGHTX>=1. STAZ records modified for birth of child (normal delivery), child's charges set to 0, sum with mother's charges, mother-baby identified by ODU, PN, MOMPN, EN, and RSNINHOS=4 or 5, BABYNORM=1 and NORMDELV=1. Description: Constructed variable indicating the hospital facility expense per stay. In Set 2, stays for newborns are not counted separately from mother's stay unless delivery was abnormal or if the infant's stay exceeded the mother's. Expense includes hospital care, room and board, laboratory charges, x-ray, and services provided by physicians not billing separately. Variable Name: EROMHO Event Source(s): EROM Variable Label: # EMERG RM VISITS RSLTNG IN HOSP ADMSS Technical Specifications: Count of EROM records w/ADMTHOSX=1 Description: Number of emergency room visits resulting in hospital admission. Variable Name: EROMHEXP Event Source(s): EROM Variable Label: EXP F/EROM VSTS RSLTNG IN HSP ADMISS ($) Technical Specifications: Sum of EXPTOTX from EROM records w/ADMTHOSX=1 Description: Total hospital expense (sum of facility expense and separate physician expense) for emergency room visits resulting in hospital admission. Variable Name: DRTEL Event Source(s): MVIS Variable Label: # PHONE VSTS W/PHYS, NOT HOSP-BASED Technical Specifications: Count of MVIS records w/MVISCLAS=1 & SEETLKMX=1 Description: Number of telephone calls to physician, not hospital-based. Variable Name: DRTELEXP Event Source(s): MVIS Variable Label: EXP F/PHONE VSTS W/PHYS, NOT HOSP-BSD ($) Technical Specifications: Sum of EXPTOTX from MVIS records w/MVISCLAS=1 & SEETLKMX=1 Description: Total expense for telephone calls to physician, not hospitalbased. Variable Name: NONDRTEL Event Source(s): MVIS Variable Label: # PHONE VSTS W/NONPHYS, NOT HOSP-BASED Technical Specifications: Count of MVIS records w/MVISCLAS=1 & SEETLKMX^=1 Description: Number of telephone calls to nonphysician, not hospital-based. Variable Name: NDRTLEXP Event Source(s): MVIS Variable Label: EXP F/PH VSTS W/NONPHYS, NOT HSP-BSD ($) Technical Specifications: Sum of EXPTOTX from MVIS records w/MVISCLAS=1 & SEETLKMX^=1 Description: Expense for telephone calls to nonphysician, not hospital-based. Variable Name: DRVISITS Event Source(s): MVIS Variable Label: # PHYSICIAN VISTS, EXCLUDING HOSP/HOME Technical Specifications: Count of MVIS records w/MVISCLAS=2 Description: Number of visits to physician, not hospital- or home-based. Variable Name: DRVISEXP Event Source(s): MVIS Variable Label: EXPENSE F/PHYS VSTS, EXCL HOSP/HOME ($) Technical Specifications: Sum of EXPTOTX from MVIS records w/MVISCLAS=2 Description: Total expense for visits to physician, not hospital- or homebased. Variable Name: DENTORTH Event Source(s): DVIS Variable Label: # DENTAL VISITS: ORTHODONTIC Technical Specifications: Count of DVIS records w/ORTHDONT=1 Description: Number of dental visits for orthodontia. Variable Name: DENTOEXP Event Source(s): DVIS Variable Label: EXP F/DENTAL VISITS: ORTHODONTIC ($) Technical Specifications: Sum of EXPTOTX from DVIS records w/ORTHDONT=1 Description: Total expense for dental visits for orthodontia. Variable Name: DRHOME Event Source(s): HVIS Variable Label: # HOME HEALTH VISITS: PHYSICIAN Technical Specifications: Sum of VISTNUMX from HVIS records w/PROVTYPE=7 Description: Number of home health physician visits. Variable Name: Event Source(s): DRHOMEXP HVIS Variable Label: EXPENSE F/HOME HLTH VSTS: PHYSICIAN ($) Technical Specifications: Sum of (VISTNUMX*VISTEXPX) from HVIS records w/PROVTYPE=7 Description: Total expense for home health physician visits. Variable Name: PMEDS Event Source(s): PMED Variable Label: # PRESCRIBED MEDICINES, INCLUDING REFILLS Technical Specifications: Sum of PRCHDATX from PMED records Description: Number of prescribed medicines, including refills. Variable Name: PMEDSEXP Event Source(s): PMED Variable Label: EXP F/PRESCRIBED MEDS, INCL REFILLS ($) Technical Specifications: Sum of EXPTOTX from PMED records. Description: Total expense for prescribed medicines, including refills. Variable Name: MEXP1EXP Event Source(s): MEXP Variable Label: EXPENSE FOR VISION ($) Technical Specifications: Sum of EXPTOTX from MEXP records w/CODEITMX=1 Description: Total expense for glasses and contact lenses. Variable Name: MEXP2EXP Event Source(s): MEXP Variable Label: EXPENSE FOR DURABLE GOODS EXCLUDING VISION ($) Technical Specifications: Sum of EXPTOTX from MEXP records w/CODEITMX not equal to: 1, 4, 5, 21, 22, 42-44, 62, 79, 91, 92 or 95 Description: Total expense for durable medical goods, including orthopedic items, hearing aids/communication items, prostheses, bathing equipment, home modifications, mattress/pads, beds, furniture, lifts and hoists, toileting equipment, feeding machines, dentures, breathing equipment, walkers/crutches, electric and nonelectric wheelchairs, canes, braces, chairlifts, blood pressure equipment, heart valves, heart monitors, OT heart items, testing and monitoring equipment, pumps, warning devices, OT hearing eqipment stimulators, and heating pads. Variable Name: MEXP3EXP Event Source(s): MEXP Variable Label: EXPENSE FOR NON-DURABLE GOODS ($) Technical Specifications: Sum of EXPTOTX from MEXP records w/CODEITMX=4, 5, 21, 22, 42-44, 62, 79, 91, 92, or 95 Description: Total expense for special medical items including diabetic items, ambulance, clothing, bandages, ostomy supplies, catheters, diapers, oxygen, OT ambulatory equipment, IV equipment and supplies, miscellaneous items, and special transportation items. Variable Name: TOTALEXP Event Source(s): STAZ, EROM, MVIS, OPAT, DENT, HVIS, PMED, MEXP Variable Label: TOTAL HEALTH CARE EXPENDITURES ($) Technical Specifications: HOSMDEXP + HOSFCEXP + EROMSEXP + DRTELEXP + NDRTLEXP + DRVISEXP + NDRVSEXP + OPDDREXP + OPDNDEXP + DENTEXP + DRHOMEXP + NDMHMEXP + PMEDSEXP + MEXP1EXP + MEXP2EXP + MEXP3EXP Description: Total health care expenditures that include the sum of all components. (Using Set 1 for hospitalization data.) Variable Name: NONDRVIS Event Source(s): MVIS Variable Label: # NONPHYS MED PROV VSTS EXCL HOSP/HOME Technical Specifications: Count of MVIS records with MVISCLAS = 3-8 Description: Number of visits to the following nonphysician medical providers who are not hospital- or home- based: chiropractor, podiatrist, optometrist, psychologist, and other medical provider (both those who do and do not work for physicians). Variable Name: NDRVSEXP Event Source(s): MVIS Variable Label: EXPENSE F/NONPHYS VSTS EXCL HSP/HME ($) Technical Specifications: Sum of EXPTOTX from MVIS records with MVISCLAS = 3-8 Description: Total expense for nonphysician medical providers, not hospitalor home-based. Variable Name: OPDDR Event Source(s): STAZ, OPAT Variable Label: # HOSP OUTPAT PHYS VSTS & O-NGHT ADMISS Technical Specifications: (Count of STAZ records w/NUMNGHTX=0) + (Count of OPAT records w/SEEDOCX < 1) Description: Number of outpatient physician visits including hospital admissions with no overnight stay. Variable Name: OPDDREXP Event Source(s): STAZ, OPAT Variable Label: EXP F/HSP OP PHYS VSTS HOSP OUTPAT VST & 0-NGT ADMSS Technical Specifications: (Sum of EXPTOTX from STAZ records w/NUMNGHTX=0) + (Sum of EXPTOTX from OPAT records w/SEEDOCX < 1) Description: Total expense for outpatient physician visits and hospital admission with no overnight stay. Variable Name: OPDNONDR Event Source(s): OPAT Variable Label: # HOSP OUTPT NONPHYSICIAN VISITS Technical Specifications: Count of OPAT records w/SEEDOCX > 1 Description: Number of nonphysician outpatient visits (i.e., chiropractors, nurses, technologists, optometrists, podiatrists, physician's �WPCD assistants, psychologists, social workers, and other medical providers). Variable Name: OPDNDEXP Event Source(s): OPAT Variable Label: EXP F/HOSP OPAT NONPHYSICIAN VSTS ($) Technical Specifications: Sum of EXPTOTX from OPAT records w/SEEDOCX > 1 Description: Total expense for nonphysician outpatient visits (i.e., chiropractors, nurses, technologists, optometrists, podiatrists, physician's assistants, psychologists, social workers, and other medical providers). Variable Name: Event Source(s): EROMS EROM Variable Label: TOTAL # EMERGENCY ROOM VISITS Technical Specifications: EROMHO + (Count of EROM records w/ADMTHOSX=2 Description: Total number of emergency room visits (including those that did and did not result in a hospital admission). Variable Name: EROMSEXP Event Source(s): EROM Variable Label: EXPENSE FOR EMERGENCY ROOM VISITS ($) Technical Specifications: EROMHEXP + (Sum of EXPTOTX from EROM records w/ADMTHOSX=2) Description: Total expense for all emergency room visits. Variable Name: NONDRMHM Event Source(s): HVIS Variable Label: # NONPHYSICIAN HOME HEALTH VSTS Technical Specifications: Count of HVIS records w/PROVTYPE NE 7 Description: Total number of non-physician home health visits (i.e., nurse/ practitioners, LPNs, physical therapists, other therapists, social workers, other nonphysician medical providers, home health aides and homemakers). Variable Name: NDMHMEXP Event Source(s): HVIS� EXP F/NONPHYSICIAN HOME HEALTH VSTS ($) Technical Specifications: Sum of (VISTNUMX* VISTEXPX) from HVIS records w/PROVTYPE NE 7 Description: Total expense for nonphysician home health visits. Variable Name: DENT Event Source(s): DVIS Variable Label: TOTAL # DENTAL VISITS Technical Specifications: DENTORTH + (Count of DENT records w/ORTHDONT NE 1) Description: Number of dental visits (orthodontic and nonorthodontic). Variable Name: Event Source(s): Variable Label: DENTEXP DVIS EXPENSE FOR DENTAL VISITS ($) Technical Specifications: DENTOEXP + (Sum of EXPTOTX from DENT records w/ORTHDONT NE 1) Description: Total expense for dental visits (orthodontic and nonorthodontic). NOTE: These estimates were made using a SAS data file and may differ slightly from estimates made using the Tape 18 EBCDIC data file or a SAS file created from the Tape 18 EBCDIC data file. COMPARISON OF ESTIMATES OF NMES HEALTH CARE UTILIZATION USING NMES PUBLIC USE TAPES 13, 14, AND 18 NOTE: to This table was originally formatted as a nine column table. Due space restrictions, the first four colums appear together followed by the remaining five columns. For NMES PUBLIC USE TAPES 13, 14, and 18 Variable DRVISITS DRTEL NONDRVIS NONDRTEL EROMS EROMHO HOSP HOSPNGT DRHOME NONDRMHM PMEDS DENT DENTORTH OPDDR OPDNONDR Unwt. #SP with Use and with Positive Weight (INCALPER) 22,828 3,208 7,171 970 5,728 1,183 3,719 3,719 172 968 20,000 13,439 937 4,747 2,771 Wt. Est. of #SP with Use and with Positive Weight (INCALPER) 161,080,390 23,506,119 52,072,475 7,162,368 37,702,370 7,017,536 24,169,932 24,169,932 969,169 5,227,676 138,958,096 101,563,130 7,490,088 30,966,001 19,330,734 NMES Public Use Tape 14 NMES PUBLIC USE TAPE 13 Total Mean Utilization � 741,869,278 39,801,274 320,275,683 10,085,806 53,424,204 8,181,324 31,225,348 204,950,141 3,841,755 254,560,170 1,231,005,708 291,766,756 39,153,609 N/A N/A 4.61 1.69 6.15 1.41 1.42 1.17 1.29 8.48 3.96 48.69 8.86 2.87 5.23 N/A N/A NMES Public Use Tape 18 VARIABLE Total Utilization DRVISITS DRTEL NONDRVIS NONDRTEL EROMS EROMHO HOSP HOSPNGT DRHOME NONDRMHM PMEDS DENT DENTORTH OPDDR OPDNONDR 741,869,278 39,801,274 320,275,683 10,085,806 53,424,204 8,181,324 31,225,348 204,950,141 3,841,755 254,560,170 1,231,005,708 291,766,756 39,153,609 81,458,388 53,659,174 Mean Utilization Tape Series# 4.61 1.69 6.15 1.41 1.42 1.17 1.29 8.48 3.96 48.69 8.86 2.87 5.23 2.63 2.78 14.5 14.5 14.5 14.5 14.5 14.5 14.4 14.4 14.2 14.2 14.1 14.3 14.3 14.5 14.5 Total Mean Utilization Utilization 741,869,278 39,801,274 320,275,683 10,085,806 53,424,204 8,181,324 31,225,348 204,950,141 3,841,755 254,560,170 1,231,005,708 291,766,756 39,153,609 81,458,388 53,659,174 4.61 � 6.15 1.41 1.42 1.17 1.29 8.48 3.96 48.69 8.86 2.87 5.23 2.63 2.78 National Medical Expenditure Survey, Household Survey, 1987. Agency for Health Care Policy and Research. NOTES: (1) For each of these utilization variables, the total utilization and mean utilization include only sample persons (SP) with a positive value for that utilization variable and with a positive value for the fullyear person-level weight (INCALPER). (2) All of these estimates were made using SAS data files and may differ slightly from estimates made using the Tape 13, 14, or 18 EBCDIC data files or SAS files created from the Tape 13, 14, or 18 EBCDIC data files. (The weighted estimates of #SP with use and with positive weight (INCALPER) were made using a SAS version of the Public Use Tape 18 data (3) file.)� The variables from Tape 18 summarizing use of outpatient services (i.e., OPDDR, OPDNONDR) have no analogous variables on Tape 13. See Section 1.6.5 of this documentation for additional details on the construction of these variables. COMPARISON OF ESTIMATES OF NMES HEALTH CARE EXPENDITURES USING NMES PUBLIC USE TAPES 14 AND 18 For NMES Public Use NMES Public Use Tape 14 Tapes 14 and 18 Variable Tape Unwt. #SP with Wt. Est. of #SP Exp and with with Exp and with Total Expenditure Mean Expenditure Series Positive Weight Positive Weight # (INCALPER) (INCALPER) DRVISEXP 14.5 22,257 157,110,773 40,124,560,016 255.39 DRTELEXP 14.5 547 3,719,705 362,471,072 97.45 NDRVSEXP 14.5 6,023 43,954,235 11,907,090,471 270.90 NDRTLEXP 14.5 170 1,212,246 71,413,612 58.91 EROMSEXP 14.5 � 4,858 32,508,850 8,855,387,184 272.40 HOSMDEXP 14.4 2,742 17,358,746 33,642,586,023 1,938.08 HOSFCEXP 14.4 3,719 24,169,932 153,881,290,216 6,366.64 DRHOMEXP 14.2 172 969,169 158,077,912 NDMHMEXP 14.2 968 5,227,676 11,406,512,846 2,181.95 PMEDSEXP 14.1 19,725 137,106,664 22,233,377,225 162.16 DENTEXP 14.3 13,439 101,563,130 29,995,670,778 295.34 163.11 DENTOEXP 14.3 937 7,490,088 7,175,349,701 957.98 MEXP1EXP 14.2 5,085 37,015,099 4,660,313,533 125.90 MEXP2EXP 14.2 1,524 9,870,875 2,267,329,594 229.70 MEXP3EXP 14.2 1,674 10,035,894 1,686,084,959 168.01 OPDDREXP 14.5 4,657 30,390,719 31,278,799,318 1,029.22 OPDNDEXP 14.5 2,721 18,978,481 11,550,006,928 608.58 TOTALEXP 14.1-5 28,704 202,169,978 364,080,971,685 1,800.87 NMES Public Use Tape 18 Variable Total Expenditure Mean Expenditure DRVISEXP DRTELEXP NDRVSEXP NDRTLEXP EROMSEXP HOSMDEXP HOSFCEXP DRHOMEXP NDMHMEXP PMEDSEXP DENTEXP DENTOEXP MEXP1EXP MEXP2EXP MEXP3EXP OPDDREXP OPDNDEXP TOTALEXP 40,124,560,016 362,471,072 11,907,090,471 71,413,612 8,855,387,184 3,642,586,002 153,881,290,290 158,077,912 11,406,512,846 22,233,374,790 29,995,670,778 7,175,349,701 4,660,313,533 2,267,329,594 1,686,084,959 31,278,801,751 11,550,005,636 364,080,970,446 255.39 97.45 270.90 58.91 272.40� 1,938.08 6,366.64 163.11 2,181.95 162.16 295.34 957.98 125.90 229.70 168.01 1,029.22 608.58 1,800.87 National Medical Expenditure Survey, Household Survey, 1987. Agency for Health Care Policy and Research. NOTES: (1) For each of these expenditure variables, the total expenditure and mean expenditure include only sample persons (SP) with a positive value for that expenditure variable and with a positive value for the fullyear person-level weight (INCALPER). (2) All of these estimates were made using SAS data files and may differ slightly from estimates made using the Tape 14 or 18 EBCDIC data files or SAS files created from the Tape 14 or 18 EBCDIC data files. (The weighted estimates of #SP with expenditures and with positive weight (INCALPER) were made using a SAS version of the Public Use Tape 18 data file.) (3) The variable TOTALEXP is the sum of the following PUF 18 expenditure variables: DRVISEXP, DRTELEXP, NDRVSEXP, NDRTLEXP, EROMSEXP, HOSMDEXP, HOSFCEXP, DRHOMEXP, NDMHMEXP, PMEDSEXP, DENTEXP, MEXP1EXP, MEXP2EXP, MEXP3EXP, OPDDREXP and OPDNDEXP.