Metric Measurements Activity
advertisement

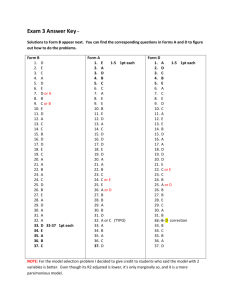
ALEKSZA NHHS H-Chemistry 2013-14 Metric Measurements Activity adapted from Berlin & Przygoda, MCC & Dr. Wilhelm, NHHS Name:________________________________________________________Score:________/_ __ Partners:_______________________________________________________________________ _______________________________________________________________________________ Introduction: The metric system of measurement is a nearly universal system which is decidedly less complex than the English system used in this county. While the basic units of the English system refer to parts of a king's body, the metric system is based on a fraction of the earth’s circumference. In addition, the metric system is divisible by units of 10. Purpose: 1. To gain familiarity with the basic metric units of length, weight, volume and temperature. 2. To gain an understanding of accuracy and precision and how these concepts apply to laboratory experiments. 3. To learn that the average of the data collected is more significant to the lab than a single data point. 4. To gain proficiency in the use of the following laboratory equipment: meter stick balances graduated glassware (cylinders, beakers, etc.) centigrade thermometers Key Terms: meter, gram, liter, centigrade, tare weight gross weight, net weight, meniscus, precision and accuracy. Materials: Metric rulers, ring stands, buret clamps, straws, tape, paper plates, graduated cylinder, buret, pipette, beakers, thermometers (digital and analog), water, calculators, electronic balance, triple beam balance, weigh boats Safety Concerns: Although all materials in this activity are considered non-hazardous, please use normal laboratory precautions such as goggles and closed-toed shoes. There is a possibility of a broken glass hazard. A. Metric Measurement (5 pts total) The prefixes for metric units are noted below. A1. The base unit for length is the __________________. (1 point) A2. The base unit for volume is the _________________. (1 point) A3. The base unit for mass is the ___________________. (1 point) A5. Define PERCISION –(1 pt) A6. Define ACCURACY – (1pt) B. Linear Measurement (11 points total) B1. Javelin/Discus Throw (8 pts) a. b. c. d. e. f. Stand on the tape placed on the floor of the lab area. Gently toss a straw or paper plate in front of you. Have a lab partner record the final location of the straw/plate. Measure the distance (in meters and centimeter) covered by the straw/plate below. Repeat steps a-d two more times Calculate your average distance. Javelin/Discus Distance1:________________________m ____________________________cm Javelin/Discus Distance2:________________________m ____________________________cm Javelin/Discus Distance3:________________________m ____________________________cm Javelin/Discus Distance(AVG):______________________m __________________________cm B2. What do you conclude about your “javelin/discus” throwing ability (use scientific terms)?(3 pts) C. Mass Measurement (17 points total) C1. Define TARE WEIGHT – (1pt) C2. Define GROSS WEIGHT – (1pt) C3. Define NET WEIGHT – (1pt) Identify the following laboratory items (1 pt each): C4.__________________________ C5. __________________________ Identify the parts of the balance: C6. C7. C8. C9. off tare C10. Word Bank: Balance Pan, Dust Cover, Off Switch, On/tare/zero switch, digital display C8. One-Hand Grab and Mass (7 pts) 1. 2. 3. 4. 5. At the weigh station, use one hand to grab as many samples as you can. Properly mass out your sample using the electronic balance. Record your data below. Repeat your measurement using the triple beam balance. Record your data below. Repeat steps 1-3 two additional times. Record your data below. Calculate your average mass of samples grabbed. Sample being tested: Mass of Samples Grab #1 Grab #2 Grab #3 Average C8b. What do you conclude about your “grabbing” skills from this activity? (3 pts) D. Volumetric Measurement (36 points total) D1. Define: MENISCUS (1pt)– D2. Identify the samples of graduated laboratory equipment below: (1 pt each) D2a________________ D2b__________________ D2c___________________ D2f._________________ Directions for reading graduated lab ware. . D2e___________________ Measuring Liquids: Accuracy & Precision (31 Points) Adapted from Honors Chemistry- Dr. Wilhelm Procedures: D3. Graduated Cylinder Using the graduated cylinders at this station (10 mL, 25 ml, 50 or 100 ml), carefully measure 9 ml of the liquid (H2O). Circle the cylinder you used for the larger volume. Transfer the liquid to a weigh boat and record the mass (g) on the electronic balance. Remember to tare the balance to account for the mass of the weigh boat prior to measuring your sample. Take triplicate measurements for each cylinder and find the average mass. Cylinder Mass (g) Average (x) 10ml 25 ml 50 or 100ml D4. Pipette Using the bulb syringe, draw in water to the pipette Carefully release the water to retain 9 mL. Transfer the 9mL to a weigh boat and record the mass. Repeat the procedure 2 more times and then find the average Graduated Pipette Mass (g) Average (x) 9 ml D5. Buret: Fill the burette with the liquid, then draw off enough liquid to fill the tip below the stopcock and bring the level of the liquid down to scale. Withdraw 9mL of water directly into a weigh boat and record the mass. Repeat and record below beret 9 ml Mass (g) Average (x) Results D6. Comparison of Five tools used to measure liquid volume (10 points – remember accuracy and precisions!) D7. Which tool was the most accurate for measuring 9 mL of liquid? Why? (3 pts) D8. Which tool was the most precise for measuring 9 mL of liquid? Why? (3pts) E. Thermal Measurement (18 points) E1. Define FARENHEIT and Note at what temperature does water boil and freeze. (2pts) – E2. Define CELCIUS and note at what temperature does water boil and freeze (2pt) – E3. Define KELVIN – (1pt) E4. Define RANKINE – (1 pt) Temperature Conversion Equations from Celsius Fahrenheit to Celsius 9 5 [°F] = [°C] × ⁄ + 32 [°C] = ([°F] − 32) × ⁄ Kelvin [K] = [°C] + 273.15 [°C] = [K] − 273.15 Rankine [°R] = ([°C] + 273.15) × ⁄ 5 9 9 5 [°C] = ([°R] − 491.67) × ⁄ 5 9 For temperature intervals rather than specific temperatures, 1 °C = 1 K and 1 ℃ = 1.8 °F E6. Identify the following: (1 pt each) E6a.__________________ E6b.___________________________ E7. Lab Water Temperature (10pts) 1. Obtain three 150ml or 250 ml beakers. Label beakers ROOM, COLD TAP and HOT TAP. 2. Fill each beaker ½ way with the type of water specified. (Run hot and cold taps for a minute or two to get a more accurate temperature. 3. Take the temperatures of the water samples using the digital of thermometers and using both Celsius and farenheit scales if available. Digital (F) Digital ( C ) Room Temp Cold Tap Warm Tap E8a. Choose 1 of the samples and manually covert the temperatures from F (actual) to C (calculated) and then one C(actual) to F (calculated). Show work! (5 pts) E5b. COMPARE your calculated values to measured values. Note any discrepancies and a probable cause. (3 pts) OVERALL: What can you conclude about precision, accuracy and laboratory measurements after conducting this activity? (5 points)