1s 2s 2p energy shell 1 shell 2 shell 3 shell 4 shell 5 3s 3p 4s 3d 4p
advertisement
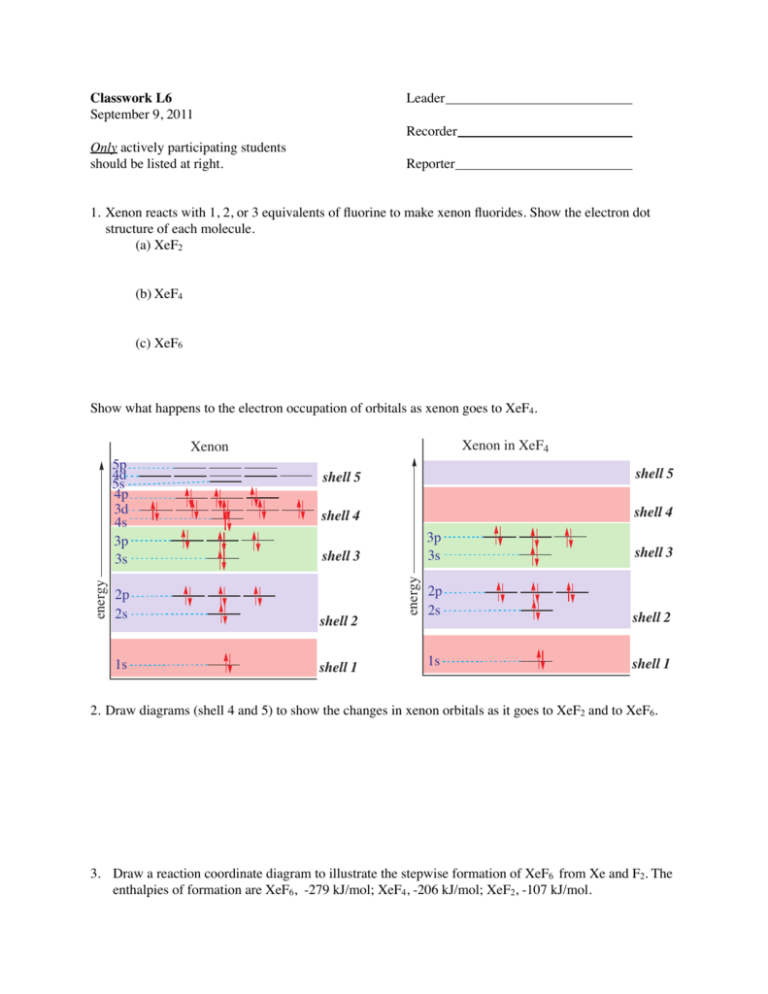
Classwork L6 September 9, 2011 Only actively participating students should be listed at right. Leader Recorder Reporter 1. Xenon reacts with 1, 2, or 3 equivalents of fluorine to make xenon fluorides. Show the electron dot structure of each molecule. (a) XeF2 (b) XeF4 (c) XeF6 Show what happens to the electron occupation of orbitals as xenon goes to XeF4. Xenon in XeF4 Xenon shell 5 shell 5 shell 4 shell 4 shell 3 2p 2s shell 2 1s shell 1 energy energy 5p 4d 5s 4p 3d 4s 3p 3s 3p 3s shell 3 2p 2s shell 2 1s shell 1 2. Draw diagrams (shell 4 and 5) to show the changes in xenon orbitals as it goes to XeF2 and to XeF6. 3. Draw a reaction coordinate diagram to illustrate the stepwise formation of XeF6 from Xe and F2. The enthalpies of formation are XeF6, -279 kJ/mol; XeF4, -206 kJ/mol; XeF2, -107 kJ/mol.





![[#IDENTITYCONNECTORS-299] SHELL scripting](http://s3.studylib.net/store/data/007586759_2-6776383e22ea2e271e255b7e6702f077-300x300.png)



