Role of VEGF in vasculogenesis and angiogenesis
advertisement

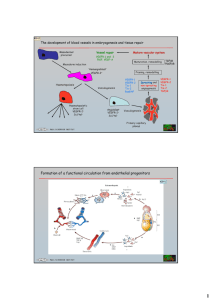
Role of VEGF in vasculogenesis and angiogenesis lecture II 14th March 2011 Angiogenesis and VEGF “history” VEGF “history” 1787 ‐ Dr John Hunter first uses the term 'angiogenesis' to describe blood vessels growth 1983 ‐ Vascular Permeability Factor (VPF), is discovered by Dr Harold Dvorak. The molecule VPF causes leaky blood vessels associated with tumors VPF was 50 000 times more potent than histamine. 1989 ‐ One of the most important angiogenic factors, vascular endothelial growth factor (VEGF), is discovered by Napoleone Ferrara and by Jean Plouet. It turns out to be identical to the molecule called Vascular Permeability Factor (VPF) discovered in 1983 by Dr. Harold Dvorak. Main proangiogenic factor Vascular Permeability Factor 1983, Dr H. Dvorak = Vascular Endothelial Growth Factor V P F vascular permeability factor endothelial cell survival factor endothelial cell proliferation endothelial cell migration V E G F 1989, Dr N. Ferrara Dr J. Plouet Blood vessel formation – various ways Carmeliet, 2005; Semenza 2003 Vasculogenesis capillaries are formed from vascular progenitor cells Vasculogenesis begins very early after the initiation of gastrulation in the mammalian embryo, with the formation of the blood islands in the yolk sac and angioblast precursors in the head mesenchyme Vasculogenesis 1. First phase • Initiated from the generation of hemangioblasts; 2. Second phase • Angioblasts proliferate and differentiate into endothelial cells 3. Third phase • Endothelial cells form primary capillary plexus In the yolk sac, these progenitors aggregate into endothelial‐lined blood islands that then fuse to generate a primary capillary plexus. The primary capillary plexus undergoes remodelling along with intra‐embryonic vessels to form a mature circulation Vasculogenesis­ blood islands YOLK SAC MESODERM ANGIOBLASTS Endothelium for vessels HEMANGIOBLASTS HEMATOPOIETIC CELLS Developing blood cells Vasculogenesis ­ initial vessel formation MESENCHYME ADVENTITIAL FIBROBLASTS Endothelium MEDIAL SMCs Endothelium Vasculogenesis – later steps ADVENTITIAL FIBROBLASTS Endothelium MEDIAL SMCs VASCULOGENESIS is prominent in establishing the vascular beds of liver, lung, & heart Hemangioblast Hemangioblast is a multipotent cell, common precursor to hematopoietic and endothelial cells. Hemangioblast was first hypothesized in 1900 by Wilhelm His. Hematopoiesis in zebrafish (Danio rerio) Expression of the flk­1 represents the earliest marker of the developing endothelial lineage during vasculogenesis SCL transcription factor is crucial for the development of blood cells and blood vessels Formation of vascular network Common vascular progenitor cells for endothelial cells and vascular smooth muscle cells Origin of endothelial and mural cells from progenitor cells Hemangioblast Blood cells Skeletal muscles Angioblast VEGF Venous endothelium Arterial endothelium Pericytes/smooth muscles PDGF­BB progenitor cells of vascular smooth muscles (various forms) VEG F ­ PDGF BB Common vascular progenitor cells (flk­1+) Origin of endothelial and mural cells from common vascular progenitor cell Flk1+ cell differentiation in serum­free culture CD31 (purple)/SMA(brown) immunostaining a, Vehicle‐treated cells. b, PDGF‐BB. c, VEGF. d, Simultaneous administration of VEGF and PDGF‐BB. Vasculogenesis in adult Vasculogenesis occurs also in adult organism Previously, postnatal vascularization was thought to occur exclusively due to angiogenesis. However, recent investigations have shown that vasculogenesis is involved in blood vessel formation also during postnatal live and the cells responsible for that are EPCs. • Classic Paradigm: Angiogenesis – Mature ECs migrate and proliferate to form new vessels • New Model: Angiogenesis + Vasculogenesis – Bone Marrow derived EPCs circulate to sites of neovascularization JCI 1999;103:1231‐1236 Endothelial progenitor cells A primitive cell made in the bone marrow (or other organs) that can enter the bloodstream and go to areas of blood‐vessel injury to help repair the damage Plasticity of adult stem cells the ability to form specialized cell types of other tissues (also called transdifferentiation) Differentiation pathways for pluripotent bone marrow stromal cells Adult progenitor cells Endothelial Progenitor Cells • 1997 Asahara et al. reported putative EPCs or angioblasts – Cells display progenitor phenotype – Differentiate into mature ECs in vitro – Participate in neovascularization when injected into ischemic animal model Science 1997;275:964-967 Endothelial Progenitor Cells DiI AcLDL uptake EPC 1:200 2h Anna Grochot­Przeczek BODIPY AcLDL uptake Anna Grochot­Przeczek Isolectin binding by EPC Lectin from Bandeira simplicifolia control isolectin Anna Grochot­Przeczek Immunohistochemical characterisation of culture­expanded EPCs 2 weeks culture Uptake of acetylated low density lipoproteins Cytoplasmic factor VIII (von Willebrand factor) tube formation on Matrigel Dzau et al., Hypertension 2005, Markers of endothelial progenitor cells CD34 VEGFR2 CD133 (prominin, AC133) CD133 is absent on mature endothelial cells and monocytic cells Mechanisms of EPCs homing and differentiation Urbich & Dimmeler, Circ Res 2004 Mobilization of vascular progenitors • Tissue ischemia results in expression of cytokines like VEGF • Recruites progenitor cells • Steady state 0.01% MNCs in blood are CEPs • Amount of circulating progenitors are increased after trauma, infectious injuries or tumour growth • 24 h after injury: 12% VEGF, VEGF­A vascular endothelial growth factor main regulator of angiogenesis, pro­angiogenic factor ‐ a dimeric glycoprotein ‐ belongs to a so‐called cysteine‐knot superfamily of growth factors ‐ one interchain disulfide bond Oloffson et al., 2000 VEGF belongs to VEGF family Endogenously expressed in mammals VEGF-A Encoded by the double stranded DNA virus, orf Ferrara et al. 2004 VEGF • VEGF (vascular endothelial growth factor, vascular permeability factor, vasculotropin) – homodimeric protein, 34‐42 kDa • produced by many types of cells (e.g. macrophages, VSMC, fibroblasts, and cancer cells) • expression is induced in response to hypoxia and proinflammatory cytokines • receptors (VEGF‐R1 and VEGF‐R2) are present mostly on endothelial cells, therefore VEGF acts specifically on endothelium (but also on neurons and Schwann cells). • VEGF‐R1 is expressed also on monocytes and vascular smooth muscle cells – their activation upregulates expression of metalloproteinases and increases cell migration. VEGF • It protects endothelial cells from apoptosis and induces their proliferation, migration, and formation of capillaries • VEGF acts protectively on neurons • VEGF is required for the normal development of embryonic vasculature, the cyclic growth of blood vessels in the female reproductive tract, and the formation of capillaries during wound repair • however, VEGF is also involved in abnormal angiogenesis, as seen in proliferative retinopathies, rheumatoid arthritis, psoriasis, and malignancies VEGF is highly conserved between species VEGF has been found in all vertebrate species : • fish (the zebrafish Danio rerio) • frogs (Xenopus laevis) • birds (Gallus gallus) • mammals The sequence and genomic organization of the vertebrate VEGF‐A genes is highly conserved between fish and mammals. Fish VEGF‐A shows 68% and 69.7% amino‐acid identity with human and mouse VEGF‐A, respectively VEGF­like proteins are present in several invertebrate species Invertebrate VEGF/VEGFR systems have been identified in fly (Drosophila melanogaster), nematode (Caenorhabditis elegans) and, most recently, in jellyfish (Podocoryne carnea). Presence of VEGF­like proteins in different animals • In the nematode Caenorhabditis elegans four possible homologs of PDGF/VEGF receptors (VER‐1 to VER‐4) and one ligand (PVF‐1) are known • PVF‐1 has the ability to bind to human receptors VEGFR‐ 1 and VEGFR‐2 and to induce angiogenesis in two model systems derived from vertebrates Control HUVEC HUVEC + VEGF HUVEC + PVF-1 Jorgensen & Mango, Nat Rev Gen 2002; Tarsitano et al. FASEB J 2006. Drosophila melanogaster respiratory (tracheal) system ‐ Branching tubular system of trachea delivers oxygen to the tissues of insects. ‐ Its development shows parallels to the angiogenesis ‐ Branchless (a homolog of mammalian FGF), PVF1, PVF2, PVF3 (homologs of mammalian VEGF/PDGF) and PVR receptor regulate the migration of early hemocytes and are necessary for formation of tracheal system. Tracheal tree of Drosophila embryo DB – dorsal branch; DT – dorsal trunk; GB – ganglionic branch; VB – visceral branch O’Farrell, J Clin Invest 2001 Organisation of VEGF gene and VEGF isoforms • The human VEGF‐A gene is characterized by a highly conserved eight exon structure, • Alternative splicing of the human VEGF‐A gene gives rise to at least five different transcripts encoding isoforms of the following lengths (in amino acids) 121, 145, 165, 189 and 206 8 VEGF-A121 8 VEGF-A145 7 8 VEGF-A165 7 8 VEGF-A189 7 8 VEGF-A206 Exons 1-5 Exons 1-5 6A1 A2 Exons 1-5 Exons 1-5 6A1 A2 Exons 1-5 6A1 A2 6B VEGF isoforms Isoform Size (amino acid) Coding exons Features VEGF-A121 121 1-5, 8 Secreted VEGF-A145 145 1-6, 8 Binds NRP2 but not NRP1; secreted VEGF-A165 165 1-5, 7, 8 The most abundant and biologically active isoform; secreted; binds NRP1 and NRP2 VEGF-A165b 165 1-5, 7, alternative exon 8 Secreted, endogenous inhibitory form of VEGF-A165 VEGF-A183 183 1-5, short exon 6, 7, 8 Sequestered in ECM but released by cleavage VEGF-A189 189 1-8 Sequestered in ECM but released by cleavage VEGF-A206 206 1-8 plus additional exon Sequestered in ECM but released by cleavage Splice variants of human VEGF 1 2-5 6a 6b 7 26 a.a. 115 a.a. 24 a.a. 17 a.a. 44 a.a. 8 6 a.a. VEGF121 VEGF145 NRP-1 VEGF165 VEGF183 VEGF189 VEGF206 signal peptide VEGF-R1 VEGF-R2 HSPGs Heparan ­Sulfate Proteoglycan Heparan­Sulfate Proteoglycan After Robinson and Stringer 2001, J Cell Science 114:853-65 Properties of VEGF isoforms VEGF121 is a soluble acid polypeptide VEGF189 and VEGF206 are highly basic and bind very strongly to heparin, thus they are completely sequestred in extra‐ cellular matrix (ECM) VEGF165 has intermediate properties: it is secreted, but significant fractions remains bound to cell surface and ECM Expression of VEGF isoforms • VEGF is produced by virtually all cell types. It is synthesized by a number of non‐malignant cells including keratinocytes, skeletal myotubes, vascular smooth muscle cells and certain endothelial cells. It is produced in big amount by different tumor cells • Most VEGF‐producing cells express VEGF121, VEGF165, VEGF189, and often VEGF183. VEGF145 and VEGF206 are seemingly restricted to cells of placental origin. • VEGF165 is most abundantly expressed, but VEGF189 is a major isoform in lungs, and both VEGF165 and VEGF189 predominate in heart. Furthermore, the relative levels of VEGF isoforms may vary during development or in response to cytokine stimulation. Not every cells express the same amounts of VEGF VEGF expression in several cell lines ­ intact cells (24 h incubation) HASMC HMEC­primary HMEC­1 HUVEC rat Müller cells 165 121 Receptors for VEGF­A Main receptors: VEGFR‐1 (Flt‐1) VEGFR‐2 (Flk1; KDR) Accessory receptors Neuropilin 1 (NRP1) Neuropilin 2 (NRP2) Storage heparan sulfate proteoglycans VEGF­A belongs to VEGF family Receptors for VEGF-A Both VEGF receptors have 7 immunoglobulin‐like domains in the extracellular domains, a single transmembrane region and a consensus tyrosine kinase sequence that is interrupted by a kinase‐insert domain. Growth factors and receptors of the VEGF family VEGF121 VEGF145 VEGF165 VEGF189 VEGF-B PlGF-1 PlGF-2 VEGF121 VEGF145 VEGF165 VEGF-C VEGF-D VEGF-E VEGF-C VEGF-D TK Sema-III Sema-E Sema-IV VEGF165 PlGF-2 VEGF-B VEGF-E Heparan-Sulfate Neuropilin-1 Proteoglycan TK VEGF-R1 VEGF145 VEGF165 VEGF189 VEGF206 VEGF-B167 VEGF-E PlGF-2 VEGF-R2 Sema-E Sema-IV VEGF165 Neuropilin-2 VEGF-R3 After Neufeld et al.. 1999, FASEB J 13:9-22 Expression of VEGF receptors ‐ endothelial cells: VEGFR‐1, VEGFR‐2, co‐receptors ‐ other cells: monocytes vascular smooth muscle cells? tumor cells? hematopoietic stem cells neuronal cells Expression of VEGF receptors is not restricted to ECs Zachary et al., 2003 Significance of VEGF and VEGF receptors has been recognized by targeting disruption of those genes in mice Knockout of VEGF is lethal in heterozygous form Yolk sac of E10.5 VEGF+/+ and VEGF +/– mouse embryos Ferrara & Alitalo, Nature Med. 2000 Effect of knockout of VEGF receptors VEGFR­1 Flt1‐/‐ mice die in utero between days 8.5 and 9.5 ‐ EC develop but do not organize into vascular channels ‐ excessive proliferation of angioblasts VEGFR­2 Flk1‐null mice die between day 8.5 and 9.5 Lack of vasculogenesis and failure to develop blood islands and organized blood vessels Semaphorin receptors – Np­1 and Np­2 ‐ form complexes with type A plexins ‐ complexes serves as signaling receptors for class‐3 semaphorins ‐ involved in axonal guidance Np­1 and Np­2 in angiogenesis ‐ binds VEGF165, VEGF‐B, PlGF‐2 ‐ knockout of Np‐1 – lethal at E12.5 ‐ overexpression of Np1‐ excessive capillary formation, dilated blood vessels extensive hemorrhage ‐ no visible abnormalities in Np‐2 knockout mice, but Np‐2‐/‐ Np1+/‐ are lethal ‐ double knockouts Np‐1‐/‐Np‐2‐/‐ died in utero at E8.5, completely avascular yolk sacs Why effect of knockouts of VEGF receptors is different? VEGF receptors – role in angiogenesis Affinity of VEGF to VEGFR1 – 16‐114 pM Affinity of VEGF to VEGFR2 – 0.4‐1 nM Effects of knockouts of VEGF genes and VEGF receptors genes Functions of the VEGF receptors family VEGFR‐1 Crucial to embryonic angiogenesis Does not appear to be critical in pathogenic angiogenesis VEGFR‐2 Mediates the majority of VEGF angiogenic effects VEGFR‐3 Found only in lymphatic endothelial cells Associated with lymph node metastasis The VEGFRs differ in their downstream signaling effects Receptor Effects VEGFR‐1 Possible “decoy receptor” effect Induction of other factors VEGFR‐2 Proliferation Migration Survival Angiogenesis VEGFR‐3 Effects mainly in lymphatic cells Angiogenic and vasculoprotective functions of VEGF ‐ vascular permeability functions ‐ endothelial cells survival factor ‐ endothelial cell proliferation ‐ endothelial cell migration ‐ inhibition of thrombosis Angiogenic and vasculoprotective functions of VEGF Mechanisms of anti­apoptotic VEGF signaling Focal adhesion kinase Phosphotydyloinositol 3 kinase AKT = PKB Zachary, Cardiovasc Res 2001 Mechanisms of mitogenic VEGF signaling phosphatydylinositol 4,5 biphosphate Phospholipase C Proteins with src homology (SH) 2 domain Diacyloglicerol+ Inositol 1,4,5 ‐trisphosphate Extraxellular signal‐regulated kinases Zachary, Cardiovasc Res 2001 Mechanisms of chemotactic VEGF signaling Zachary, Cardiovasc Res 2001 VEGF level has to be tightly regulated during development and postnatal life Embryonic development is disrupted by modest increases in VEGF gene expression Miquerol L, Langille BL, Nagy A. Development, 2000: 127:3941-6 2­3 fold overexpression is deletorious to embryonic development Enlarged hearts Embryos died between E12.5 and E14.5 Too high and unbalanced expression of VEGF after gene delivery using adenoviral vectors A and B. Note prominent tissue edema and new blood vessel formation. C. Note also a prominent leakage of plasma protein complexes from locally hyperpermeable ear vessels. The effect of different isoforms of VEGF on angiogenesis Vessel formation and sprouting angiogenesis in embronyic bodies in response to the different VEGF ligands Formation of peripheral vascular plexus in two‐dimensional EB cultures, visualized by anti‐CD31 immunostaining (red), was induced by 1 nmol/L VEGF‐A165, but not by VEGFA165b,VEGF‐A121, VEGF‐A145, or vehicle. Kawamura et al. Cancer Res. 2008 Vessel formation and sprouting angiogenesis in embronyic bodies in response to the different VEGF ligands Vascularization of Matrigel plugs in nude mice. Plugs were fixed and stained to detect CD31 on endothelial cell (red) and α‐SMA (ASMA) on pericytes (green) by immunofluorescent detection. Kawamura et al. Cancer Res. 2008 VEGF­A165b inclusion of VEGF‐A165b led to invasion of endothelial cells into the Matrigel, but the cells failed to organize into vessels and also failed to attract pericytes endothelial cells pericytes Kawamura et al. Cancer Res. 2008 VEGF­A121 In the VEGF‐A121–containing Matrigel plugs, occasional vessel structures were seen, which lacked branch points and a pericyte coat endothelial cells pericytes Kawamura et al. Cancer Res. 2008 VEGF­A145 In VEGF‐A145–containing Matrigel plugs, branching, pericyte‐clad vessels were seen but to a much lesser extent than in the VEGF‐A165–containing Matrigel plugs endothelial cells pericytes Kawamura et al. Cancer Res. 2008 VEGF­A165 Inclusion of VEGF‐A165 induced abundant vascularization of the Matrigel with richly branched, pericyte‐covered vessels endothelial cells pericytes !VEGF165 is the crucial isoform! Kawamura et al. Cancer Res. 2008 How to assess the role of different VEGF isoforms, if the knockout of the gene is lethal? Conditional knockouts of genes This strategy is based on a tissue‐specific inactivation of the gene of interest. This can be achieved by means of a Cre recombinase, that catalyzes site‐specific recombination of DNA between loxP sites. A Cre recombinase is an enzyme that deletes the DNA fragment located between the two recombinase‐specific (LoxP) sites. A mouse bearing the recombinase‐specific sites (introduced by homologous recombination in Embryonic Stem cells) is bred with a mouse expressing the recombinase (generated by homologous recombination or transgenesis). The tissue‐specific expression of the recombinase allows the inactivation of the gene of interest only in the tissue where the recombinase is expressed. Cre­driven conditional expression of genes Transgenic animals, in which the target gene is flanked by Lox sequences, must also express Cre recombinase Thus, they have to be cross‐bred with mice expressing Cre. The expression of Cre can be: 1. Tissue specific – Cre gene is driven by the tissue specific promoter, eg. heart, liver etc. 2. Conditionally induced – Cre gene is driven by the inducible promoter, eg. tetracycline‐induced or IFN‐α induced VEGF is required for growth and survival in neonatal mice One allele of VEGF deleted in exon 3 Gerber et al., 1999 VEGF is required for growth and survival in neonatal mice 1. 38% mortality at day 7 in mice without VEGF (its synthesis was blocked from day 3); 2. Liver changes ‐ smaller hepatocytes, immature sinusoids, increased extramedullary hematopoiesis and almost complete absence of Flk‐1 positive endothelial cells; 3. Similar effects as after targeted knockouting of VEGF were obtained when mice were treated with anti‐VEGF antibodies Targeting of VEGF isoform­specific alleles Stalmans et al., JCI 2002 Impaired retinal vascular development in VEGF120/120 and VEGF188/188 mice Stalmans et al., JCI 2002 Arteriolar and venular patterning in retinas of mice selectively expressing VEGF isoforms The present study investigates the distinct role of the different VEGF isoforms in retinal vascular development. Retinal vascular development was normal in VEGF164/164 mice. In contrast, VEGF120/120 mice exhibited severe vascular defects, with impaired venous and severely defective arterial vascular development in the retina. VEGF188/188 mice had normal venous development, but aborted arterial outgrowth. Stalmans et al., JCI 2002 Effect of conditional knockout of VEGF164 on myocardial angiogenesis Capillary density increases 300% in VEGF+/+ hearts (filled bars) but not in VEGF120/120 hearts (stippled bars). There were fewer α‐actin stained coronary vessels per section in VEGF120/120 hearts (stippled bars) than in VEGF+/+ hearts (filled bars). Carmeliet et al., 1999 Viability of VEGF­isoform mice VEGF120/120 – half neonates died shortly after births because of cardiorespiratory distress; the other died within 2 weeks after birth, in part due to impaired myocardial angiogenesis resulting in cardiac failure VEGF164/164 – were normal VEGF188/188 – half of embryos died in utero ‐ surviving gain less weigth, were less fertile Take­home messages VEGF (VEGF‐A) is a key mediator of vasculogenesis, angiogenesis and arteriogenesis VEGF is generated in the form of several isforms, being the results of alternative splicing The most common and the most active and crucial isoform is VEGF165 VEGF exerts its activity by binding to its receptors: VEGFR1, VEGFR2 and co‐ receptors: neuropilin 1 & 2. VEGFR2 is the key receptor, mediating the majority of actions of VEGF. VEGFR1 is a decoy receptor, playing an important role in modulating VEGF activity during development