ΔG, E, and K
advertisement

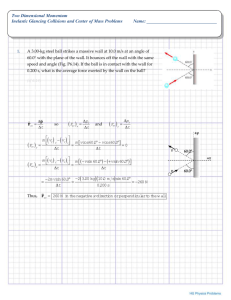
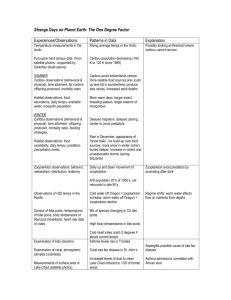
ΔG, E, and K Gibbs Free Energy, Cell Potential and Equilibrium Constant ΔG = ΔH ‐ TΔS if ΔH < 0 and ΔS > 0, ΔG < 0 at all temps and reaction is prod. fav’d at all temps; if ΔH < 0 and ΔS < 0, ΔG < 0 only at low temps and rx. is prod. fav’d only at low temps; if ΔH > 0 and ΔS > 0, ΔG < 0 only at high temps and rx. is prod. fav’d only at high temps; if ΔH > 0 and ΔS < 0, ΔG > 0 at all temps and rx. is react. fav’d at all temps. T = ΔH / ΔS ΔG = ΔG + RT ln Q ΔG = ‐ RT ln K and therefore, K < 1 ΔG > 0 rx. is react. fav’d K > 1 ΔG < 0 rx. is prod. fav’d K = 1 ΔG = 0 rx. is neither prod. fav’d nor react. fav’d ΔG = ‐ nFEcell and therefore, if ΔG < 0, E > 0 and rx. is prod. fav’d ΔG > 0 , E < 0 and rx. is react. fav’d E°cell = E°cathode ‐ E°anode or E°cell = E°reduction + E°oxidation Ecell = (RT / nF) ln K Ecell = Ecell ‐ (RT / nF) ln Q be able to calculate ΔG under non‐standard conditions be able to calculate either the equilibrium constant or the change in Gibbs Free Energy, given the other be able to calculate standard cell potentials given the potentials of the half‐cell reactions ln X = 2.303 log X this is the Nernst Equation, allowing you to calculate cell potential at non‐standard conditions (or even Ecell from non‐standard cell conditions and E cell) and all other stuff from the ACIDS and BASES summary sheet and all other stuff from the ACIDS and BASES summary sheet in case you should need to know if log X = Y X = 10Y in case you should need to know if ln X = Y X = eY or X = 2.718Y in case you should need to know [H3O+] = 10‐pH and [OH‐] = 10‐pOH pH = ‐ log [H3O+] and pOH = ‐log [OH‐] be able to calculate ΔH , ΔS and ΔG at standard temp and be able to calculate ΔG at non‐standard temps be able to calculate the temperature at which the spontaneity of a reaction reverses direction