chemical formulas study guide
advertisement
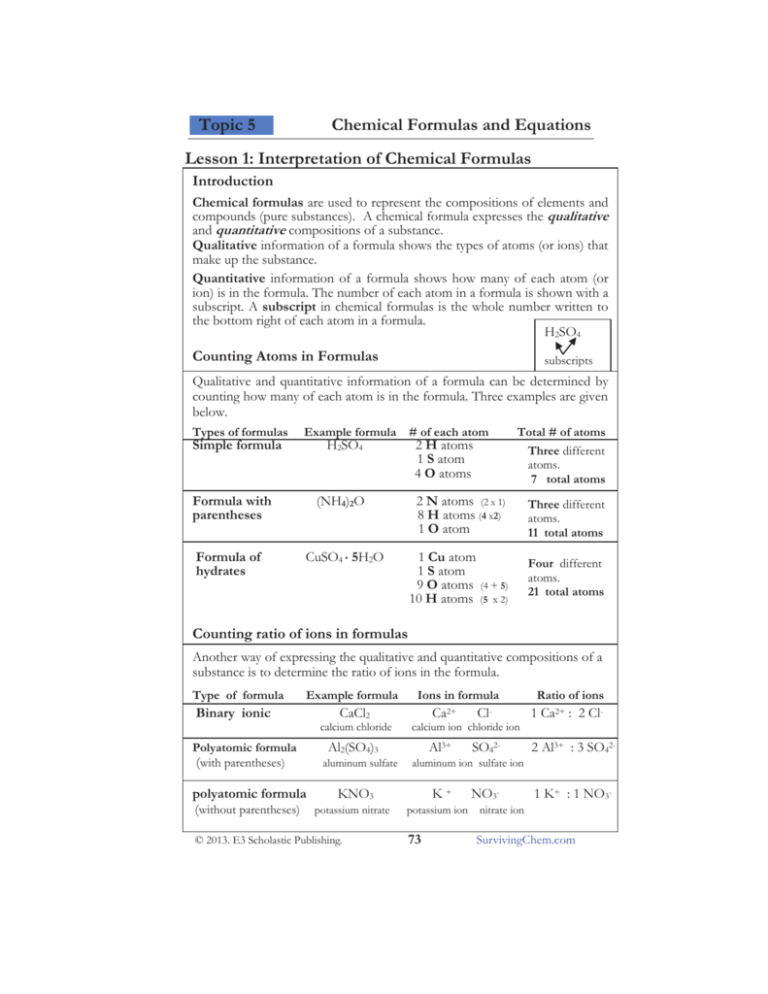
Topic 5 Chemical Formulas and Equations Lesson 1: Interpretation of Chemical Formulas Introduction Chemical formulas are used to represent the compositions of elements and compounds (pure substances). A chemical formula expresses the qualitative and quantitative compositions of a substance. Qualitative information of a formula shows the types of atoms (or ions) that make up the substance. Quantitative information of a formula shows how many of each atom (or ion) is in the formula. The number of each atom in a formula is shown with a subscript. A subscript in chemical formulas is the whole number written to the bottom right of each atom in a formula. H2SO4 Counting Atoms in Formulas subscripts Qualitative and quantitative information of a formula can be determined by counting how many of each atom is in the formula. Three examples are given below. Types of formulas Simple formula Formula with parentheses Formula of hydrates Example formula # of each atom H2SO4 (NH4)2O CuSO4 . 5H2O Total # of atoms 2 H atoms 1 S atom 4 O atoms Three different atoms. 7 total atoms 2 N atoms (2 x 1) 8 H atoms (4 x2) 1 O atom Three different atoms. 11 total atoms 1 Cu atom 1 S atom 9 O atoms (4 + 5) 10 H atoms (5 x 2) Four different atoms. 21 total atoms Counting ratio of ions in formulas Another way of expressing the qualitative and quantitative compositions of a substance is to determine the ratio of ions in the formula. Type of formula Binary ionic Example formula CaCl2 calcium chloride Polyatomic formula (with parentheses) Ions in formula Ca2+ Ratio of ions 1 Ca2+ : 2 Cl- calcium ion chloride ion Al2(SO4)3 aluminum sulfate Cl- Al3+ SO42- 2 Al3+ : 3 SO42- aluminum ion sulfate ion polyatomic formula KNO3 (without parentheses) potassium nitrate K+ NO3- 1 K+ : 1 NO3- potassium ion nitrate ion © 2013. E3 Scholastic Publishing. 73 SurvivingChem.com Topic 5 Chemical Formulas and Equations Lesson 2: Types of Chemical Formulas There are three types of chemical formulas that are used to show compositions of substances. In this lesson, you will learn about molecular formulas, empirical formulas, and structural formulas. Types of Formulas: A molecular formula is a formula showing the true composition of a known substance. A structural formula is a formula showing how atoms of a substance are bonded together. An empirical formula is a formula in which the elements are in the smallest whole-number ratio. Examples of the three formulas are shown below: ethane Molecular C2H6 H Structural Empirical H2O H H–C –C–H H water O / \ H H H CH3 H2O C2H6 is reduced to CH3 by dividing each subscript of the formula by 2 (Greatest Common Factor of 2 and 6) C6H12 O6 can be reduced to CH2O by dividing each subscript of the formula by 6 (Greatest Common Factor of 6,12, and 6) © 2013. E3 Scholastic Publishing. 74 SurvivingChem.com Topic 5 Chemical Formulas and Equations Lesson 3 – Chemical Nomenclature Introduction: There are millions of known chemical substances, and many more are being discovered. Chemical nomenclature refers to the systematic rules for naming and writing formulas of chemical substances. The International Union of Pure and Applied Chemistry (IUPAC) is an organization that makes recommendations as to how chemical substances are named. In this lesson, you will learn how to apply IUPAC rules to writing formulas and names for compounds in different classes of inorganic compounds. Chemical Formulas A chemical formula is correctly written for a known substance when both the qualitative and quantitative information of the formula are both correct. This to say that: . Element (or ion) symbols in a formula must all be correct for the substance. . Subscripts of the elements (or ions) in a formula must be in the correct ratio. Correct subscripts in a formula allow the sum of charges in the formula to equal zero. All compound formulas must be neutral. Steps to writing chemical formulas for ionic compounds When the IUPAC name for an ionic compound is given, use the steps below to write its correct formula. Step 1: Write the correct ion symbols for the chemical name. Use the Periodic Table to get the correct ion symbol for an element. Use Table E to get the correct polyatomic ion symbol. Always put parentheses around polyatomic atoms. Ex. (SO4)2Step 2: Criss-cross charge values so one becomes the subscript for the other. Step 3: Clean up formula after criss-crossing by: Reducing subscripts that are reducible to empirical form. For polyatomic ion compounds: Do not change subscripts of the polyatomic ion Remove parentheses if subscript outside parentheses is a 1 Keep parentheses if subscript outside parentheses is greater than a 1 Steps shown above and on the next pages are to ensure that your final (correct) formula has the correct element symbols and correct subscripts for the compound name that is given. If you can write correct formulas without going through all these steps, you should do so. © 2013. E3 Scholastic Publishing. 75 SurvivingChem.com Topic 5 Chemical Formulas and Equations Writing Formulas for Ionic Compounds Binary ionic compounds have formulas that are composed of two different elements: a metal and a nonmetal. IUPAC names of binary compounds always end with –ide. Examples: Calcium bromide, Aluminum sulfide, and Zinc oxide Example 1: Calcium bromide Step 1 Ca2+ Br 1– Ca2+ Br1– criss-crossing charge values Step 2 Ca1 Br2 Step 3 (formulas) Aluminum Sulfide Zinc Oxide Al3+ S2– Al3+ S2– Zn2+ O2– Zn2+ O2– Al2 S3 Ca Br2 Zn2 O2 Al2 S3 ZnO Ionic Compounds Containing a Polyatomic Ion Polyatomic ions are composed of two or more atoms with an excess charge. Reference Table E lists some common polyatomic ions . IUPAC names of polyatomic ions typically end with -ate or –ite. Examples of compounds containing a polyatomic ion are: sodium nitrate, calcium sulfite, and ammonium oxide. Example 2: Sodium Nitrate Step 1 Step 2 Step 3 (formulas) +1 1– Calcium Sulfite Ammonium Oxide 2– (NH4)1+ O2– Na+1 (NO3)1– Ca2+ (SO3)2– (NH4)1+ O2– Na1 (NO3)1 Ca2 (SO3)2 (NH4)2 O 1 NaNO3 CaSO3 (NH4)2 O Na (NO3) Ca 2+ (SO3) Ionic Compounds Containing an Atom with Multiple Oxidation #s. The stock system nomenclature uses Roman numerals in parentheses to distinguish names of compounds produced by different positive oxidation states of an atom. Examples of Stock system naming and the interpretation of a Roman numeral in names are given below. Iron (II) chloride (II) indicates a compound of a +2 iron. Iron (III) chloride Nitrogen (IV) oxide (III) indicates a compound of a +3 iron (IV) indicates a compound of a +4 nitrogen Example 3: Iron (II) chloride ↓ Iron (III) chloride ↓ Fe2+ Cl1– Fe3+ Cl1– FeCl2 FeCl3 © 2013. E3 Scholastic Publishing. 76 Nitrogen (IV) oxide ↓ N4+ O2– NO2 SurvivingChem.com Topic 5 Chemical Formulas and Equations Writing IUPAC Names for Ionic Compounds The IUPAC name is correctly written for a given formula when all of the following are represented correctly. . Atoms and/or ions in the formula are named correctly. . Name ending , if necessary, is applied correctly. . Roman numeral, if necessary, is correctly used. Binary ionic compounds contain just two elements: a metal and nonmetal. Examples are ZnCl2, CaO and Al2N3. IUPAC naming of binary compounds involves changing the nonmetal ending to –ide. The metal name is never changed. Example 5: ZnCl2 Zinc Chlorine CaO Calcium Oxygen Al2 N3 Aluminum Nitrogen Zinc Chloride Calcium oxide Aluminum nitride Compounds containing a polyatomic ion generally contain three or more elements. Examples are Mg2(PO4)3 , NH4NO3 and NH4Cl. When naming compounds containing a polyatomic ion, no change should be made to the name of the polyatomic ion (See Table E) or to the name of the metal. Example 6: Mg2(PO4)3 Magnesium phosphate NH4NO3 Ammonium nitrate NH4Cl Ammonium chloride Compounds containing an element with multiple oxidation numbers must be named using the Stock System. In each of the three formulas below, the first element has multiple (+) oxidation numbers (See the Periodic Table to confirm). A Roman numeral (in parentheses) is used in naming to identify which positive charge of the element formed the compound. Example 7: Sn F4 Tin (IV) fluoride N2O Nitrogen (I) oxide Fe3(PO4)2 Iron (II ) phosphate In some formulas (like the three above) the subscript of the second symbol in each formula is used as the Roman Numeral (or the +charge) value in the ( ). In some formulas (like the two below), the +charge value must be determined mathematically by following steps given in the box to the left. Example 8: Assign (– ) charge value. CrN2 MnSO4 3– Cr N2 2 x 3- = - 6 Mn (SO4)2– 1 x 2- = -2 Multiply ( – ) charge by subscript to get total (– ) in formula . +6 +2 Determine total (+) needed to make charges = 0 Use + charge as Roman numeral Chromium (VI) nitride Manganese (II) sulfate in ( ) to name the formula. © 2013. E3 Scholastic Publishing. 77 SurvivingChem.com Topic 5 Chemical Formulas and Equations Writing Formulas and Naming Covalent (Molecular) Substances Molecular (covalent) compounds are composed only of nonmetal atoms. Binary molecular compounds, which contain two different nonmetals, are commonly named with IUPAC recommended prefixes. A prefix in a chemical name indicates how many of the nonmetal atom is in a given compound. The table below lists prefixes for naming covalent compounds. Number of atom 1 2 3 4 5 Prefix monoditritetrapenta- Number of atom 6 7 8 9 10 Prefix hexaheptaoctanonadeca- Writing formulas for covalent substances: Follow rules and examples below . Prefixes are interpreted into subscripts for the elements. . Absence of a prefix indicates that there is just one of that atom. . No criss-crossing or reducing of formula into empirical form is necessary. Example 9 Molecular name Carbon dioxide 1C Interpretation 2O CO2 Formula Carbon monoxide 1C dinitrogen monoxide 1O 2N CO 1O N2O Writing names for covalent substances: Follow rules and examples below. . Subscripts are interpreted into prefixes for the elements . No prefix is used when there is just one of the first nonmetal atom (see PCl3) . The “a” or “o” of a prefix is dropped if the addition of the prefix resulted in a name having two vowels next to each other (see N2O5) In N2O5, the a in pentaoxide is dropped, and the formula is correctly named pentoxide. . Name ending for the second nonmetal atom must be changed to -ide Example 10: PCl3 1 Phosphorus 3 Chlorine N2O5 2 nitrogen 5 oxygen Phosphorus trichloride dinitrogen pentoxide © 2013. E3 Scholastic Publishing. 78 H2S 2 hydrogen 1 sulfur dihydrogen monosulfide SurvivingChem.com